Archive \ Volume.11 2020 Issue 1
Comparative Study Between Conventional Trans-Arterial Chemoembolization (TACE) And Drug Eluting Bead TACE Regarding Tumour Response and Liver Function Tests
Amgad M. Elsheikh1*, Mohamed I. Teama1, Afify F. Afify2, Mohamed H. Abowarda1, Hosam N.Almassry1
1 Radiodiagnosis Department, Faculty of Medicine, Zagazig University, Sharkia, Egypt. 2 Internal medicine department, Faculty of Medicine, Zagazig University, Sharkia, Egypt.
Abstract
Objectives: To compare tumour response and liver function tests changes after conventional TACE with lipidol versus DEB-TACE in the treatment of non-resectable HCC. Patients and methods: Prospective non randomized comparative clinical trial was performed for patients receiving TACE at interventional radiology unit in Radiodiagnosis department in Zagazig university hospitals. Forty patients were included in this study, 16 patients were treated with drug eluting beads TACE and 24 patients were treated with conventional TACE. Results: Follow up triphasic CT was performed 1 month after the procedure, we found that complete response was 6 cases (25 %) in c TACE group, and 4 cases (25%) in drug eluting bead TACE group, Partial response was achieved in 11 cases (45.8%) in c TACE group, and in 8 cases (50 %) in DEBs-TACE group, Cases with stable disease were 5 cases (20.8%) in c TACE group, and it was 3 cases (18.7%) in DEBs-TACE group, progressive disease was noted in two cases (8.3 %) in c TACE group, and one case (6.2 %) in drug eluting TACE group. Mean albumin value in c TACE and DEBs TACE patients was averaged 3.5 and 3.6 gm/dl before treatment and 3.4 and 3.39 gm/dl after treatments, respectively. Mean value of total bilirubin in cTACE was averaged 1.6 mg/dl after treatment vs. 1.3mg/dl before treatment, whereas, in DEB TACE group reached 1.7 mg/dl after treatment vs.1.36mg/dl before treatment. Mean value of ALT was 31 and 32 u/L in c TACE and DEB TACE groups respectively before treatment, it was 32 and 36 u/L in c TACE and DEB TACE groups respectively after treatment. Mean value of AST was 40 and 38 u/L in c TACE and DEB TACE groups respectively before treatment, it was 43 and 45 u/L in c TACE and DEB TACE groups respectively after treatment. Conclusion: There were no significant differences between two groups regarding tumour response after 1 month. There was slight reduction in albumin level in post treatment groups, and there was elevation in total bilirubin, ALT and AST, however no significant difference was seen between two groups.
Keywords: c TACE, DEBs TACE, HCC, LFTs, response
INTRODUCTION
Hepatocellular carcinoma (HCC) is a standout amongst the most widely recognized cancers with a yearly rate of around 750000 cases for every year around the world. [1] The dominant part of patients are diagnosed at middle of the road or progressed clinical stages, which rejects them from possibly curative treatment, for example, resection, liver transplantation, or local ablation [2]. As indicated by the Barcelona Clinic Liver Cancer grouping (BCLC), transarterial chemoembolization (TACE) is the standard treatment for patients with intermediate stage HCC [2] .The great bulk of HCC patients are not candidates for liver resection because they have advanced disease with widespread tumour growth, significantly impaired functional reserve of the cirrhotic liver and / or existing portal hypertension, possibly with associated thrombosis of the portal vein [3]. Transarterial chemoembolization (TACE) currently represents the standard treatment for patients with advanced non-resectable HCC [4]. In spite of a number of publications, there is still no agreement on the choice of chemotherapeutic agents and on the TACE treatment regime [5]. Drug-eluting bead transarterial chemoembolization (DEB-TACE) has been extensively commercially available since 2006. Since then, DEB-TACE has become the de facto standard in a lot of centers worldwide, many investigators believe it to be more beneficial than conventional TACE with lipidol (cTACE) [6]. Drug-eluting bead is a new drug that enables embolization of vessels supplying hypervascularized malignant tumours with concurrent administration of a local, controlled, sustained dose of a chemotherapeutic agent to the tumour [7]. In this clinical trial we compare conventional TACE versus DEB TACE regarding tumour response and liver function tests changes.
MATERIAL AND METHODS:
Study design
The study was approved by our Institutional Review Board. A prospective non randomized comparative clinical trial was performed for patients receiving TACE at interventional radiology unit in Radiodiagnosis Department in Zagazig University Hospitals between January 2018 and December 2019.Tumour response rate and liver function tests were assessed.
Study Population
Through 2 years, selected patients, who attended to Radiodiagnosis Department and met the inclusion criteria for transarterial chemo-emolization of hepato-cellular carcinoma were included in the study.
Forty patients were included in this study, 16 patients were treated with Drug eluting beads TACE and 24 patients were treated with conventional TACE.
Inclusion criteria:
- Any age group and sex.
- Patients suffering from heptocellular carcinoma and are candidate for TACE:
A) Tumor size is usually more than 5 cm.
B) Patients with multinodular tumors without vascular invasion or extrahepatic spread.
C) Patients with early stage HCC when surgical options or percutaneous ablation are contra-indicated or not suitable.
D) Liver functional reserve is a critical component for careful patient selection, patients should present with relatively well preserved liver function (mostly child pugh A or B without ascites).
Exclusion criteria:
- Patients with complete portal vein thrombosis.
- Patients with contra-indication to contrast media administration.
- Patients who are candidate for radio-frequency ablation or surgical resection.
- Patients who are unwilling to complete the study.
- Patients with suspected unavailability throughout the study.
- Arterio-portal shunts.
- Vascular anatomy precluding correct catheter placement.
- Presence of collateral vessels pathways potentially endangering normal territories during embolization.
- Patients with non HCC malignancies (e.g. cancer colon metastasis).
- Patients with extra-hepatic spread.
All included patients were subjected to:
- Complete history taking.
- Pre-procedural laboratory evaluation including : (LFTs, RFTs, CBC, INR, α-fetoprotein level, Viral markers).
- Imaging including:
- Pre-procedural triphasic computed tomography.
- Angiogram (Before the procedure).
- Procedure:
- Conventional TACE: We used lipidol and adriamycin.
- DEB TACE: We used drug-eluting beads (Hepashere).
- Angiogram (After the procedure)
- Follow-up triphasic CT within 1 month.
- Post-procedural laboratory evaluation including: LFTs and α-fetoprotein level.
Approval was obtained from ethical Zagazig University Institutional Review Board (IRB).
Patients were divided into cTACE group and DEBS-TACE group according to the TACE regimen they received.
Patients were diagnosed with HCC and they had a triphasic CT imaging within one month prior to their TACE procedure.
Patients were diagnosed with HCC either by the classic radiological features of a hepatic lesion with arterial phase enhancement and portal venous phase washout or by biopsy.
Biopsy was performed in lesions with equivocal radiologic features, we injected local anaesthesia first (usually lidocaine), then we used US guidance to introduce 18 gauge needle biopsy, then biopsy was taken and sent for histopathological analysis . Liver function was quantified via the Child-Pugh score.
The decision to perform TACE to patients with HCC was made by a consensus of a multi-disciplinary liver tumor board at our Zagazig University Hospitals, that includes medical oncologists, hepatologists, interventional radiologists and surgeons.
TACE protocol
The interventional radiologists used either a Lipiodol-Doxorubicin based or drug eluting beads-Doxorubicin mixture for TACE procedures. The cTACE mixture consisted of 10 ml of Lipiodol (Guerbet, Paris, France) mixed with 50 mg of Doxorubicin. This was followed by administration of Gelfoam particles if needed to achieve complete stasis in the target artery. The DEBS-TACE mixture consisted of (30-60 um) HepaSpheres expanding microspheres (BioSphere Medical, France) that were loaded with 50 mg of Doxorubicin according to the manufacturer’s protocol. It was reaching to size (120-240 um) in hydrate state. The end point for TACE for both mixtures was stasis of flow in the target artery.
Most of TACE procedures were performed with the catheter placed in the segmental artery or sub-segmental hepatic arterial branches before the administration of the TACE mixture. However, placement of the catheter in the right or left hepatic artery (lobar TACE) was sometimes performed when it was clinically indicated such as in multifocal HCC or invasive HCC without a dominant mass.
Post-TACE HCC tumor response:
Follow up with triphasic CT imaging was obtained within 1 month after TACE. Tumor response rate after TACE was categorized according to the four categories of the mRECIST: complete response, partial response, stable disease or progressive disease.
Complete response (CR) is defined as complete disappearance of tumor arterial enhancement. Partial response (PR) is defined as at least 30% reduction in the sum of the diameter of arterial enhancement in reference to baseline diameter. Progressive disease (PD) is defined as at least 20% increase in the sum of the longest diameter of the lesions in reference to baseline diameter. Stable disease (SD) is defined as a response that does not categorized as partial response or progressive disease category.
Liver function tests are recorded 1 week after the procedure.
Statistical analysis:
- Patient demographics, clinical history, laboratory data, and cross sectional imaging data were collected. Pre-TACE CT variables including number of lesions, size of tumors, and total axial diameter of the 3 largest tumors in the case of multifocal HCC were analyzed. Tumor response from post-TACE CT according to mRECIST criteria were recorded, liver function tests were recorded 1 week after the procedure.The data was analyzed with proper statistical tests.
- The collected data was coded, entered, presented, and analyzed by computer using a data base software program, Statistical Package for Social Science (SPSS) version 20.
- Qualitative data was represented as frequencies and percents.
- Chi square (X2) was used to detect relation between different qualitative variables.
- For quantitative variables mean and standard deviation were computed.
- Independent t-test (t) was used for detection of difference between different quantitative variables.
- The results were considered statistically significant and highly statistical significant when the significant probability (P value) was < 0.05*.
Case (1):
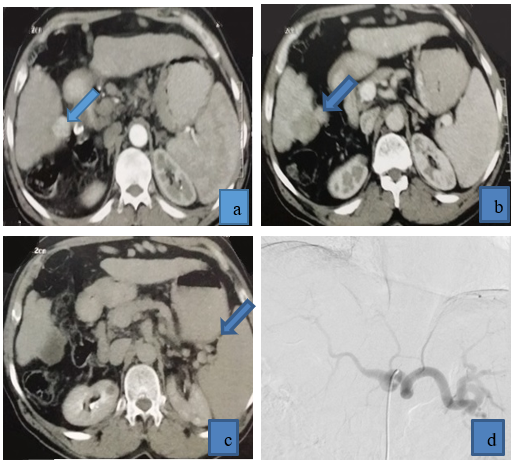
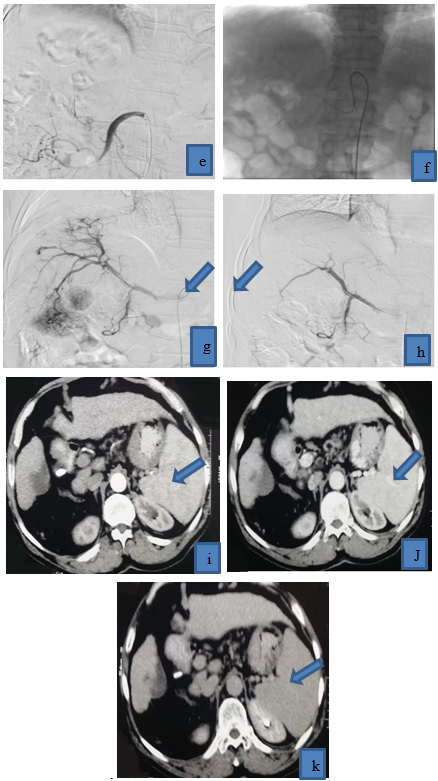
Figure 1: 64 years old patient came with multifocal right lobe HCC, he received drug eluting beads TACE achieving complete response in follow up CT, (a) triphasic CT demonstrated right lobe HCC that was enhanced in arterial phase, (b) venous phase: HCC displayed washout, (c) delayed phase: HCC displayed washout. (d) Copra head catheter failed to access celiac trunk due to kink at its origin, (e) Superior mesenteric artery angiography was done to exclude replaced right hepatic artery due to tumor location in segment V and VI, (f) we tried to access by Simmons catheter to reach to pathological vessels, (g) preoperative angiography revealed tumor blush and pathological circulation in right lobe HCC, (h) post-operative angiography revealed disappearance of pathological blush (technical success), post-operative triphasic CT revealed cystic degeneration of HCC (complete response), with no residual activity in arterial phase(i), venous phase (j),and delayed phase(k).
Case (2)
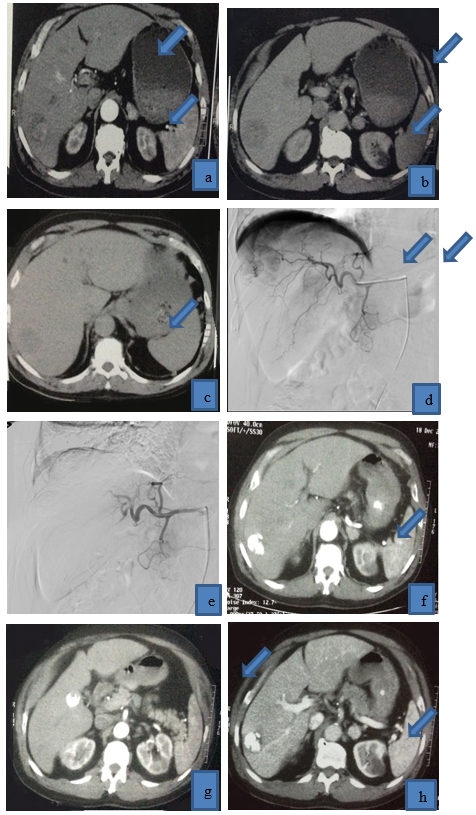

Figure 2: 62 years old patient presented with multifocal right lobe HCC, (a) it displayed heterogenous enhancement in arterial phase, (b),(c) it displayed washout in venous and delayed phases, (d) selective catheterization of right hepatic artery revealed tumor blush and pathological circulation of HCC, and then lipidol and Adriamycin were injected, (e) post-operative angiogram revealed disappearance of pathological circulation (technical success), post-operative triphasic CT was done (f ) (g) arterial phase, (h)(i) venous phase, (j)(K) delayed phases, HCC is completely filled with lipidol with reduction of its size, achieving complete response, no residual tumoral activity.
RESULTS:
Table (1) shows no significant difference between two groups regarding age or sex.The HCC were more common in old age males. HCC also were more common in cirrhotic liver. Smoking history was similar between both groups. Tumor characteristics were nearly similar between two groups, multifocal HCC were more common than unifocal HCC in both groups.Tumor size was ranging from 3 cm to 8 cm with mean diameter 5.5 cm in first group, and it was ranging from 2.4 cm to 8 cm with mean diameter 5.2 cm in second group, two cases with partial portal vein thrombosis (30%) were done by using conventional TACE, and one case with partial portal vein thrombosis (25%) was done by DEBs-TACE.
HCC occur on top viral infection to the liver, HBV and HCV, Cases with HCV are more common than cases with HBV in both groups. We carefully selected our patients as they were with BCLC 0, A, and B.
In conventional TACE, one patient was with BCLC 0 (4.1%), 4 patients were with BCLC A (16.6%), and 19 patients were with BCLC B (79.1%).
In drug eluting beads TACE, 5 patients were with BCLC A (31.25%), and 11 patients were with BCLC B (68.75%).
Table (2) We found that complete response was 6 cases (25%) in c TACE group, and 4 cases (25 %) in drug eluting bead TACE group, partial response was achieved in 11 cases (45.8%) in c TACE group, partial response was achieved in 8 cases (50 %) in DEBs-TACE group, Cases with stable disease were 5 cases (20.8%) in cTACE group, and it was 3 cases (18.7 %) in drug eluting bead TACE group, Progressive disease was noted in two cases (8.3%) in c TACE group, and one case (6.2%) in drug eluting TACE group.
Objective response rate (ORR) was defined as percentage of patients who achieved complete response or partial response, for example ORR in follow up after 1month , it was 70.8 % in c TACE group, and it was 75 % in drug eluting bead TACE.
There were no significant differences between two groups regarding tumor response after 1 month.
|
Table 1: patients’ characteristics |
|||
|
Item |
Conventional TACE (n=24) |
DEBs-TACE (n= 16) |
b P-value |
1-Age at TACE (years) (mean) 2-Sex (male/female) 3-Smoking (Yes/no) 4-Liver cirrhosis (Yes/no) |
64.2 ±3.1 20/4 12/12 22/2 |
65.1±3.0 13/3 9/7 14/2 |
a 0.368 0.865 0.69 0.66 |
Child paugh A/B |
18/6 |
12/4 |
1.0 |
1-Number of tumors (Unifocal/multifocal) 2-Largest tumor. (cm) (mean) 3-Partial portal vein thrombosis (yes/no) 4- Bilobar HCC (Yes/no) |
11/13 5.5 ±2.5 (3-8) 2/22 13/11 |
7/9 5.2 ±2.8 (2.4-8) 1/15 8/8 |
0.64 a 0.725 0.806 0.79 |
1-Hepatitis B 2-Hepatitis C |
3 21 |
2 14 |
1.0 |
-BCLC 0 -BCLC A -BCLC B -BCLC C -BCLC D |
1 4 19 0 0 |
0 5 11 0 0 |
0.58 |
-ECOG 0 -ECOG 1 -ECOG 2 |
15 5 4 |
10 5 1 |
0.53 |
a Independent t-Test b Chi square test (X2)
|
Table 2: tumor response after 1 month |
|||
|
Variables |
Conventional TACE (n=24) |
DEBs-TACE (n= 16) |
P value |
|
Complete response (CR) |
6 (25%) |
4 (25%) |
0.99 |
|
Partial response (PR) |
11 (45.8 %) |
8 (50 %) |
|
|
Stable disease (SD) |
5 (20.8 %) |
3 (18.7%) |
|
|
Progressive disease (PD) |
2 (8.3 %) |
1 (6.2%) |
|
Chi square test (X2)
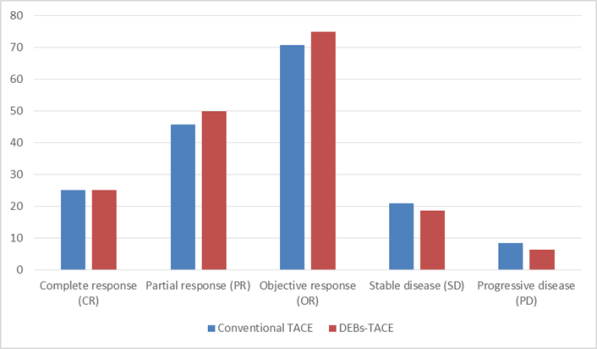
Diagram showing tumour response after 1 month
Mean value of albumin was 3.5 gm/dl in c TACE group before treatment, and it was 3.4 gm/dl after treatment, mean value of albumin in DEBs TACE group was 3.6 gm/dl before treatment, it was 3.39 gm/dl after treatment. Mean value of total bilirubin was 1.3 mg/dl in c TACE group before treatment, it was 1.6 mg/dl after treatment. Mean value of total bilirubin was 1.36 mg/dl in DEB TACE group before treatment, it was 1.7 mg/dl after treatment. Mean value of ALT was 31 and 32 u/L in c TACE and DEB TACE groups respectively before treatment, it was 32 and 36 u/L in c TACE and DEB TACE groups respectively after treatment. Mean value of AST was 40 and 38 u/L in c TACE and DEB TACE groups respectively before treatment, it was 43 and 45 u/L in c TACE and DEB TACE groups respectively after treatment.
There was slight reduction in albumin level in post treatment groups, and there was elevation in total bilirubin, ALT and AST, however no significant difference was seen between two groups.
|
Table 3: liver function tests in two groups |
||||||
|
Parameter |
Baseline |
After 1 week of treatment |
||||
|
|
C- TACE |
DEBs-TACE |
P-Value |
C-TACE |
DEBs-TACE |
P-value |
|
1)Albumin(gm/dl) |
3.5±0.9 |
3.6±0.92 |
0.72 |
3.4±0.82 |
3.39±0.83 |
0.97 |
|
2)Total bilirubin(mg/dl) |
1.3±0.12 |
1.36±0.13 |
0.161 |
1.6±0.3 |
1.7±0.4 |
0.372 |
|
3)ALT (u/L) |
31 ±9.1 |
32 ±9.2 |
0.73 |
32±8.9 |
36±9.1 |
0.176 |
|
4) AST (u/L) |
40.0±5.2 |
38.0±4.8 |
0.227 |
43.0±4.7 |
45.0±5.2 |
0.212 |
Independent t-Test
DISCUSSION:
The choice of TACE regimen and the value of using the more expensive drug eluting beads is still debated, and is often based on the doctor`s bias or his experience, despite the proposed benefits of DEBs, there is limited and conflicting data on the efficacy of DEBs-TACE compared to conventional TACE.
We performed this prospective comparative study between conventional TACE and DEBs-TACE in order to overcome bias towards using one of the two regimens, our data revealed no significant difference between the two groups regarding tumour response and liver function tests changes.
In our clinical study, there was no significant difference between two groups regarding patients characteristics, no significant difference in age, sex, smoking history or liver cirrhosis between two groups.
Both groups were nearly have same percentage of patients in child paugh stage A and child paugh stage B, no significant difference was found regarding number of tumours, largest tumour size and bilobar versus unilobar tumours.
HCC occur on top viral infection to the liver, HBV and HCV, Cases with HCV were more common than cases with HBV in both groups. We carefully selected our patients, patients were with BCLC 0, A, and B, we excluded BCLC C & D to minimize occurrence of complications as possible as we can.
There were no significant differences between two groups regarding tumor response after 1 month.
A comparative study between c TACE and DEB-TACE was done by Li et al. ,who found that no difference of complete response between DEB-TACE group (14.3%) and c TACE group (5.1%, P=0.167), however , the objective response rate (ORR) was increased in DEB-TACE group (73.8%) than that in c TACE group (41%, P=0.003). [8]
We found that our results were partially agree with that study regarding no difference in complete response in between two groups, however objective response rate in our study was also of no significant difference in contrary to their study, this difference may be due to their sample size which was (81) HCC patients, (42) with DEB-TACE, (39) with c TACE, this number was double our sample size, it can reveal small differences in ORR in between groups.
M. D. Ferrer Puchol et al., did a similar comparative study and found no difference in complete response in between two groups of patients. [9]
van Malenstein H et al., did a similar comparative study and found no difference in complete response in between two groups of patients, and he also did his study on small sample size, (14) patients did c TACE and (16) patients did DEB-TACE.[10]
Despite theoretical advantages of DEB-TACE, it is still controversial in clinical practice as to whether DEB-TACE is superior to c TACE in regard to overall survival and treatment response, recently reported meta-nalysis showed that the two modalities represent comparable results, suggesting an absence of difference in tumor response between DEB-TACE and cTACE [11]
In comparison with cTACE, DEB-TACE facilitates higher concentrations of drugs within the target tumor and lower systemic concentrations. Despite the theoretical advantages of DEB-TACE, it is still controversial in several clinical studies as to whether DEB-TACE is superior to cTACE in terms of efficacy. However, it seems that DEBTACE shows at least similar clinical outcomes and less adverse events than cTACE. [11]
Liver function tests after TACE procedure show reduction in albumin level, and elevation in total bilirubin , ALT and AST, theses changes are similar to clinical trial done in Menoufiya university in Egypt , that was done by Kohla MAS ., et al. [12]
No significant difference in liver function tests changes in between two groups in our study, This result was similar to another study done in china comparing LFTs between two groups of patients (conventional TACE versus DEBS-TACE), they concluded that no significant difference in between two groups regarding ALT, AST and total bilirubin levels, however there is slight reduction in albumin level in DEBs-TACE group compared with c TACE group, they justified this reduction because they included patients with liver surgery in their clinical trial, percentage of these patients are higher in DEB-TACE group, so this affected liver function and resulted in less albumin production in DEB-TACE group.[8]
In our study , albumin level shows no significant difference between two groups because we excluded patients with liver surgery from our clinical trial , so this factor made no effect on our results.
Strength in our study that it is real life comparative prospective clinical trial, it enables evaluation of the two methods in clinical practice with Egyptian patients.
There is still several limitations to this study as:
- It was a single center experience .
- The sample size in our study was relatively small which might reduce statistical power.
- The follow up time was rather short to observe survival profit of patients, which should be prolonged in the future studies.
- Lack of randomization , which may lead to some selection bias.
ACKNOWLEDGMENT:
I would like to thank professor Dr. Mohamed I. Teama, professor of Radiodiagnosis, Zagazig University, for his help and great support in the completion of this work.
CONCLUSION:
Trans-arterial chemoembolization is considered the standard management for intermediate-stage HCC. Moreover, it is recognized as the most common bridging therapy offered to patients on a waiting list for liver transplantation.
Variations in TACE protocols are vast. A wide range of chemotherapeutic mixtures have been reported in the literature like bland embolization, chemoembolization and chemoperfusion. Initially, transarterial therapy for HCC was performed using bland embolization. Conventional TACE has been used for years with the gold standard mixture composed of Lipiodol mixed with chemotherapeutic agents , most commonly Doxorubicin . Recently, Drug eluting beads are appearing as new drug .
Drug eluting beads have been introduced and marketed as a better chemotherapeutic carrier and embolic agent. The mechanism of DEBS action is achieved through prolongation of the contact time between chemotherapeutic agents and tumor cells with sustained slower release of chemotherapeutic agents, which leads to lower peak plasma concentrations/systemic toxicity and theoretically greater tumor toxicity. Despite these proposed benefits of DEBS, there is limited and conflicting data on the efficacy of DEBS-TACE compared to cTACE.
The current study represents comparative clinical trial between two groups of patients, 1st group represents convential TACE , we used (lipidol as embolizing agent mixed with Doxorubicin), 2nd group represents DEBs-TACE, we used drug eluting beads, Statistical analysis of our results show no significant difference between two groups regarding tumour response and liver function tests changes (baseline and 1 week after the operation).
Using drug eluting beads can minimize complications rate , however its cost is still limiting factor as many patients can not afford it, we recommend using DEBs-TACE in large capsulated lesions , as this can result in better tumour response.
Further multi-center randomized controlled clinical trials with larger sample size are recommended to address the tumour response, complications rate, liver status and changes in α-fetoprotein level to overcome current study limitations.
Conflict of interest:
The author declared that there is no conflict of interest.
Highlights/Teaching points:
- TACE is treatment of choice for intermediate stage HCC.
- Variations in TACE protocol are vast. Some interventional radiologists use conventional TACE with lipidol and Adriamycin, others use drug eluting bead TACE with hepasphere particles.
- This clinical trial compare tumour response and liver function tests changes between two groups of patients received TACE.
- We concluded that no difference in tumour response in follow up CT after 1 month, between two groups, also there is no difference in liver function tests changes in between two groups.
Abbreviations:
c TACE: conventional trans-arterial chemoembolization,
DEB TACE: drug eluting beads trans-arterial chemoembolization,
HCC: hepatocellular carcinoma,
CR: complete response,
PR: partial response,
SD: stable disease,
PD: progressive disease,
LFTs: liver function tests.
REFERENCES
- Jemal A, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a Cancer Journal for Clinicians.2011;61(2):69-90.
- European Association for Study of Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012; 48(5): 599-641.
- Llovet JM, Burroughs A, Bruix J: Hepatocellular carcinoma. Lancet, 2003; 362: 1907-17.
- Bruix J, Sherman M: Management of hepatocellular carcinoma. Hepatology, 2005; 42: 1208-36.
- Llovet JM, Bruix J: Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology, 2003; 37: 429-42.
- Bargellini I, Florio F, Golfieri R, Grosso M, Lauretti DL, Cioni R. Trends in utilization of transarterial treatment for hepatocellular carcinoma: results of a survey by the Italian society of interventional radiology. Cardiovascintervent Radiol. 2014; 37(2): 438-44.
- Lewis AL, Gonzalez MV, Leppard SW et al: Doxorubicin eluting beads: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med, 2007; 18: 1691-99.
- Li H, Wu F, Duan M, et al. Drug- eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety.Medicine (2019)98:21.
- Ferrer Puchol MD, La Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma.Radiologia.2011; 53(3):246-253.
- Malenstein H, Maleux G, Vandecaveye V,et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie 2011;34:368-376.
- Song JE and Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol 2017 june 28;9(18): 808-814.
- Kohla MAS, Abu Zeid MI, Al-Warraky M, et al. Predictors of hepatic decompensation after TACE for hepatocellular carcinoma. BMJ open Gastro 2015;2:e000032.doi:10.1136/bmjgast-2015-000032.
INTRODUCTION
The liver is among the most complex and pivotal organs in human body [1]. Hepatocellular carcinoma (HCC) is a standout amongst the most widely recognized cancers with a yearly rate of around 750000 cases every year around the world. [2, 3] The dominant part of patients are diagnosed in the middle of the road or progressed clinical stages, which rejects them from possibly curative treatment, for example, resection, liver transplantation, or local ablation [4, 5]. As indicated by the Barcelona Clinic Liver Cancer grouping (BCLC), transarterial chemoembolization (TACE) is the standard treatment for patients with intermediate-stage HCC [4]. The great bulk of HCC patients are not candidates for liver resection because they have advanced disease with widespread tumor growth, a significantly impaired functional reserve of the cirrhotic liver, and/or existing portal hypertension, possibly with associated thrombosis of the portal vein [6]. Transarterial chemoembolization (TACE) currently represents the standard treatment for patients with advanced non-resectableHCC [7]. Despite several publications, there is still no agreement on the choice of chemotherapeutic agents and the TACE treatment regime [8]. Drug-eluting bead transarterial chemoembolization (DEB-TACE) has been extensively commercially available since 2006. Since then, DEB-TACE has become the defacto standard in a lot of centers worldwide, many investigators believe it to be more beneficial than conventional TACE with lipiodol (cTACE) [9]. A drug-eluting bead is a new drug that enables the embolization of vessels supplying hyper vascularized malignant tumors with concurrent administration of a local, controlled, sustained dose of a chemotherapeutic agent to the tumor [10]. In this clinical trial, we compare conventional TACE versus DEB-TACE regarding tumor response and liver function test changes.
Keywords: c TACE, DEBs TACE, HCC, LFTs, response
MATERIALS AND METHODS:
Study design
The study was approved by our Institutional Review Board. A prospective nonrandomized comparative clinical trial was performed for patients receiving TACE at interventional radiology unit in Radiodiagnosis Department in Zagazig University Hospitals between January 2018 and December 2019. Tumour response rate and liver function tests were assessed.
Study Population
Through 2 years, selected patients, who attended to Radiodiagnosis Department and met the inclusion criteria for transarterial chemo-embolization of hepatocellular carcinoma were included in the study.
Forty patients were included in this study, 16 patients were treated with Drug-eluting beads TACE and 24 patients were treated with conventional TACE.
Inclusion criteria:
- Any age group and sex.
- Patients suffering from hepatocellular carcinoma and are a candidate for TACE:
- Tumor size is usually more than 5 cm.
- Patients with multinodular tumors without vascular invasion or extrahepatic spread.
- Patients with early-stage HCC when surgical options or percutaneous ablation are contra-indicated or not suitable.
- A liver functional reserve is a critical component for careful patient selection, patients should present with relatively well-preserved liver function (mostly child-pugh A or B without ascites).
Exclusion criteria:
- Patients with complete portal vein thrombosis.
- Patients with contra-indication to contrast media administration.
- Patients who are a candidate for radio-frequency ablation or surgical resection.
- Patients who are unwilling to complete the study.
- Patients with suspected unavailability throughout the study.
- Arterio-portal shunts.
- Vascular anatomy precluding correct catheter placement.
- Presence of collateral vessel pathways potentially endangering normal territories during embolization.
- Patients with non HCC malignancies (e.g. cancer colon metastasis).
- Patients with an extra-hepatic spread.
All included patients were subjected to:
- Complete history taking.
- Pre-procedural laboratory evaluation including : (LFTs, RFTs, CBC, INR, α-fetoprotein level, Viral markers).
- Imaging including:
- Pre-procedural triphasic computed tomography.
- Angiogram (Before the procedure).
- Procedure:
- Conventional TACE: We used lipiodol and adriamycin.
- DEB-TACE: We used drug-eluting beads (Hepashere).
- Angiogram (After the procedure)
- Follow-up triphasic CT within 1 month.
- Post-procedural laboratory evaluation including LFTs and α-fetoprotein level.
Approval was obtained from the ethical Zagazig University Institutional Review Board (IRB).
Patients were divided into the cTACE group and DEBS-TACE group according to the TACE regimen they received.
Patients were diagnosed with HCC and they had a triphasic CT imaging within one month before their TACE procedure.
Patients were diagnosed with HCC either by the classic radiological features of a hepatic lesion with arterial phase enhancement and portal venous phase washout or by biopsy.
A biopsy was performed in lesions with equivocal radiologic features, we injected local anesthesia first (usual lidocaine), then we used US guidance to introduce 18 gauge needle biopsy, then biopsy was taken and sent for histopathological analysis. Liver function was quantified via the Child-Pugh score.
The decision to perform TACE to patients with HCC was made by a consensus of a multi-disciplinary liver tumor board at our Zagazig University Hospitals, which includes medical oncologists, hepatologists, interventional radiologists, and surgeons.
TACEprotocol
The interventional radiologists used either a Lipiodol-Doxorubicin based or drug-eluting beads-Doxorubicin mixture for TACE procedures. The cTACE mixture consisted of 10 ml of Lipiodol (Guerbet, Paris, France) mixed with 50 mg of Doxorubicin. This was followed by the administration of Gelfoam particles if needed to achieve complete stasis in the target artery. The DEBS-TACE mixture consisted of (30-60 um) HepaSpheres expanding microspheres (BioSphere Medical, France) that were loaded with 50 mg of Doxorubicin according to the manufacturer’s protocol. It was reaching to size (120-240 um) in a hydrated state. The endpoint for TACE for both mixtures was stasis of flow in the target artery.
Most of TACE procedures were performed with the catheter placed in the segmental artery or sub-segmental hepatic arterial branches before the administration of the TACE mixture. However, placement of the catheter in the right or left hepatic artery (lobar TACE) was sometimes performed when it was clinically indicated such as in multifocal HCC or invasive HCC without a dominant mass.
Post-TACE HCC tumor response:
Follow up with triphasic CT imaging was obtained within 1 month after TACE. Tumor response rate after TACE was categorized according to the four categories of the mRECIST: complete response, partial response, stable disease, or progressive disease.
Complete response (CR) is defined as complete disappearance of tumor arterial enhancement. Partial response (PR) is defined as at least a 30% reduction in the sum of the diameter of arterial enhancement about baseline diameter. Progressive disease (PD) is defined as at least a 20% increase in the sum of the longest diameter of the lesions about baseline diameter. Stable disease (SD) is defined as a response that does not categorize as a partial response or progressive disease category.
Liver function tests are recorded 1 week after the procedure.
Statistical analysis:
- Patient demographics, clinical history, laboratory data, and cross-sectional imaging data were collected. Pre-TACE CT variables including several lesions, size of tumors, and total axial diameter of the 3 largest tumors in the case of multifocal HCC were analyzed. Tumor response from post-TACE CT according to mRECIST criteria was recorded, liver function tests were recorded 1 week after the procedure. The data were analyzed with proper statistical tests.
- The collected data were coded, entered, presented, and analyzed by computer using a database software program, Statistical Package for Social Science (SPSS) version 20.
- Qualitative data were represented as frequencies and percents.
- Chi-square (X2) was used to detect the relationship between different qualitative variables.
- For quantitative variables mean and standard deviation were computed.
- Independent t-test (t) was used for the detection of a difference between different quantitative variables.
- The results were considered statistically significant and highly statistically significant when the significant probability (P-value) was < 0.05*.
Case (1):


Figure 1: 64 years old patient came with multifocal right lobe HCC, he received drug-eluting beads TACE achieving a complete response in follow up CT, (a) triphasic CT demonstrated right lobe HCC that was enhanced in arterial phase, (b) venous phase: HCC displayed washout, (c) delayed phase: HCC displayed washout. (d) Copra head catheter failed to access celiac trunk due to kink at its origin, (e) Superior mesenteric artery angiography was done to exclude replaced right hepatic artery due to tumor location in segment V and VI, (f) we tried to access by Simmons catheter to reach to pathological vessels, (g) preoperative angiography revealed tumor blush and pathological circulation in right lobe HCC, (h) post-operative angiography revealed disappearance of pathological blush (technical success), post-operative triphasic CT revealed cystic degeneration of HCC (complete response), with no residual activity in the arterial phase(i), venous phase (j), and delayed phase(k).
Case (2)

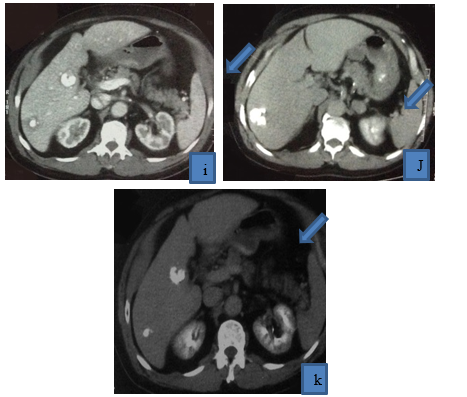
Figure 2: 62 years old patient presented with multifocal right lobe HCC, (a) it displayed heterogeneous enhancement in arterial phase, (b),(c) it displayed washout in venous and delayed phases, (d) selective catheterization of right hepatic artery revealed tumor blush and pathological circulation of HCC, and then lipiodol and Adriamycin were injected, (e) postoperative angiogram revealed disappearance of pathological circulation (technical success), post-operative triphasic CT was done (f ) (g) arterial phase, (h)(i) venous phase, (j)(K) delayed phases, HCC is filled with lipiodol with reduction of its size, achieving a complete response, no residual tumoral activity.
RESULTS:
Table (1) shows no significant difference between the two groups regarding age or sex. The HCC was more common in old age males. HCC also were more common in cirrhotic liver. Smoking history was similar between both groups. Tumor characteristics were nearly similar between two groups, multifocal HCC was more common than unifocal HCC in both groups. Tumor size was ranging from 3 cm to 8 cm with mean diameter 5.5 cm in the first group, and it was ranging from 2.4 cm to 8 cm with mean diameter 5.2 cm in the second group, two cases with partial portal vein thrombosis (30%) were done by using conventional TACE, and one case with partial portal vein thrombosis (25%) was done by DEBs-TACE.
HCC occurs on top viral infection to the liver, HBV, and HCV, Cases with HCV are more common than cases with HBV in both groups. We carefully selected our patients as they were with BCLC 0, A, and B.
In conventional TACE, one patient was with BCLC 0 (4.1%), 4 patients were with BCLC A (16.6%), and 19 patients were with BCLC B (79.1%).
In drug-eluting beads TACE, 5 patients were with BCLC A (31.25%), and 11 patients were with BCLC B (68.75%).
Table (2) We found that complete response was 6 cases (25%) in c TACE group, and 4 cases (25 %) in drug-eluting bead TACE group, partial response was achieved in 11 cases (45.8%) in c TACE group, partial response was achieved in 8 cases (50 %) in DEBs-TACE group, Cases with stable disease were 5 cases (20.8%) in cTACE group, and it was 3 cases (18.7 %) in drug-eluting bead TACE group, Progressive disease was noted in two cases (8.3%) in c TACE group, and one case (6.2%) in drug-eluting TACE group.
Objective response rate (ORR) was defined as the percentage of patients who achieved a complete response or partial response, for example, ORR in follows up after 1month, it was 70.8 % in c TACE group, and it was 75 % in drug-eluting bead TACE.
There were no significant differences between the two groups regarding tumor response after 1 month.
|
Table 1: patients’ characteristics |
||||
|
Item |
Conventional TACE (n=24) |
DEBs-TACE (n= 16) |
b P-value |
|
1-Age at TACE (years) (mean) 2-Sex (male/female) 3-Smoking (Yes/no) 4-Liver cirrhosis (Yes/no) |
64.2 ±3.1 20/4 12/12 22/2 |
65.1±3.0 13/3 9/7 14/2 |
a 0.368 0.865 0.69 0.66 |
|
|
Child-Pugh A/B |
18/6 |
12/4 |
1.0 |
|
|
1-Number of tumors (Unifocal/multifocal) 2-Largest tumor. (cm) (mean) 3-Partial portal vein thrombosis (yes/no) 4- Bilobar HCC (Yes/no) |
11/13 5.5 ±2.5 (3-8) 2/22 13/11 |
7/9 5.2 ±2.8 (2.4-8) 1/15 8/8 |
0.64 a 0.725 0.806 0.79 |
|
|
1-Hepatitis B 2-Hepatitis C |
3 21 |
2 14 |
1.0 |
|
|
-BCLC 0 -BCLC A -BCLC B -BCLC C -BCLC D |
1 4 19 0 0 |
0 5 11 0 0 |
0.58 |
|
|
-ECOG 0 -ECOG 1 -ECOG 2 |
15 5 4 |
10 5 1 |
0.53 |
|
aIndependent t-Test b Chi-square test (X2)
|
Table 2: tumor response after 1 month |
|||
|
Variables |
Conventional TACE (n=24) |
DEBs-TACE (n= 16) |
P-value |
|
Complete response (CR) |
6 (25%) |
4 (25%) |
0.99 |
|
Partial response (PR) |
11 (45.8 %) |
8 (50 %) |
|
|
Stable disease (SD) |
5 (20.8 %) |
3 (18.7%) |
|
|
Progressive disease (PD) |
2 (8.3 %) |
1 (6.2%) |
|
Chi-square test (X2)
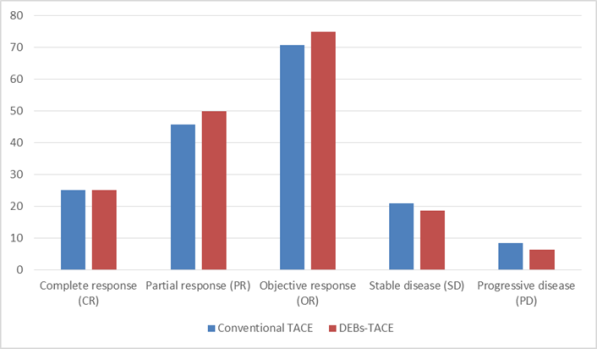
Diagram showing tumor response after 1 month
The mean value of albumin was 3.5 gm/dl in c TACE group before treatment, and it was 3.4 gm/dl after treatment, the mean value of albumin in the DEBs TACE group was 3.6 gm/dl before treatment, it was 3.39 gm/dl after treatment. The mean value of total bilirubin was 1.3 mg/dl in c TACE group before treatment, it was 1.6 mg/dl after treatment. The mean value of total bilirubin was 1.36 mg/dl in the DEB-TACE group before treatment, it was 1.7 mg/dl after treatment. The mean value of ALT was 31 and 32 u/L in c TACE and DEB-TACE groups respectively before treatment, it was 32 and 36u/L in c TACE and DEB-TACE groups respectively after treatment. The mean value of AST was 40 and 38 u/L in c TACE and DEB-TACE groups respectively before treatment, it was 43 and 45 u/L in c TACE and DEB-TACE groups respectively after treatment.
There was a slight reduction in albumin level in post-treatment groups, and there was an elevation in total bilirubin, ALT, and AST, however, no significant difference was seen between the two groups.
|
Table 3: liver function tests in two groups |
||||||
|
Parameter |
Baseline |
After 1 week of treatment |
||||
|
|
C- TACE |
DEBs-TACE |
P-Value |
C-TACE |
DEBs-TACE |
P-value |
|
1)Albumin(gm/dl) |
3.5±0.9 |
3.6±0.92 |
0.72 |
3.4±0.82 |
3.39±0.83 |
0.97 |
|
2)Total bilirubin(mg/dl) |
1.3±0.12 |
1.36±0.13 |
0.161 |
1.6±0.3 |
1.7±0.4 |
0.372 |
|
3)ALT (u/L) |
31±9.1 |
32±9.2 |
0.73 |
32±8.9 |
36±9.1 |
0.176 |
|
4) AST (u/L) |
40.0±5.2 |
38.0±4.8 |
0.227 |
43.0±4.7 |
45.0±5.2 |
0.212 |
Independent t-Test
DISCUSSION:
The choice of TACE regimen and the value of using the more expensive drug-eluting beads is still debated and is often based on the doctor`s bias or his experience, despite the proposed benefits of DEBs, there is limited and conflicting data on the efficacy of DEBs-TACE compared to conventional TACE.
We performed this prospective comparative study between conventional TACE and DEBs-TACE to overcome the bias towards using one of the two regimens, our data revealed no significant difference between the two groups regarding tumor response and liver function tests changes.
In our clinical study, there was no significant difference between the two groups regarding patients' characteristics, no significant difference in age, sex, smoking history, or liver cirrhosis between two groups.
Both groups nearly had the same percentage of patients in child-pugh stage A and child-pugh stage B, no significant difference was found regarding several tumors, the largest tumor size and bilobar versus unilobartumours.
HCC occurs on top viral infection to the liver, HBV, and HCV, Cases with HCV were more common than cases with HBV in both groups. We carefully selected our patients, patients were with BCLC 0, A, and B, we excluded BCLC C & D to minimize the occurrence of complications as possible as we can.
There were no significant differences between the two groups regarding tumor response after 1 month.
A comparative study between c TACE and DEB-TACE was done by Li et al., who found that no difference of complete response between DEB-TACE group (14.3%) and c TACE group (5.1%, P=0.167), however, the objective response rate (ORR) was increased in DEB-TACE group (73.8%) than that in c TACE group (41%, P=0.003).[11]
We found that our results partially agreed with that study regarding no difference in complete response in between two groups, however objective response rate in our study was also of no significant difference in contrary to their study, this difference may be due to their sample size which was (81) HCC patients, (42) with DEB-TACE, (39) with c TACE, this number was double our sample size, it can reveal small differences in ORR in between groups.
M. D. Ferrer Puchol et al., did a similar comparative study and found no difference in complete response in between two groups of patients. [12].
van Malenstein Het al. did a similar comparative study and found no difference in complete response between two groups of patients, and he also did his study on small sample size, (14) patients did c TACE, and (16) patients did DEB-TACE.[13]
Despite theoretical advantages of DEB-TACE, it is still controversial in clinical practice as to whether DEB-TACE is superior to c TACE concerning overall survival and treatment response, recently reported meta-analysis showed that the two modalities represent comparable results, suggesting an absence of difference in tumor response between DEB-TACE and Ctace [14]
In comparison with cTACE, DEB-TACE facilitates higher concentrations of drugs within the target tumor and lower systemic concentrations. Despite the theoretical advantages of DEB-TACE, it is still controversial in several clinical studies as to whether DEB-TACE is superior to cTACE in terms of efficacy. However, it seems that DEB-TACE shows at least similar clinical outcomes and less adverse events than cTACE.[14]
Liver function tests after TACE procedure show reduction in albumin level, and elevation in total bilirubin, ALT, and AST, these changes are similar to clinical trials done in Menoufiya University in Egypt, that was done by KohlaMAS ., et al.[15]
No significant difference in liver function tests changes in between two groups in our study, This result was similar to another study done in china comparing LFTs between two groups of patients (conventional TACE versus DEBS-TACE), they concluded that no significant difference in between two groups regarding ALT, AST and total bilirubin levels, however, there is a slight reduction in albumin level in DEBs-TACE group compared with c TACE group, they justified this reduction because they included patients with liver surgery in their clinical trial, percentage of these patients are higher in DEB-TACE group, so this affected liver function and resulted in less albumin production in DEB-TACE group.[11]
In our study, albumin level shows no significant difference between the two groups because we excluded patients with liver surgery from our clinical trial, so this factor made no effect on our results.
Strength in our study that it is a real-life comparative prospective clinical trial, it enables evaluation of the two methods in clinical practice with Egyptian patients.
There are still several limitations to this study as:
- It was a single-center experience.
- The sample size in our study was relatively small which might reduce statistical power.
- The follow-up time was rather short to observe the survival profit of patients, which should be prolonged in future studies.
- Lack of randomization, which may lead to some selection bias.
ACKNOWLEDGMENT:
I would like to thank Professor Dr. Mohamed I. Teama, professor of Radiodiagnosis, Zagazig University, for his help and great support in the completion of this work.
CONCLUSION:
Trans-arterial chemoembolization is considered the standard management for intermediate-stage HCC. Moreover, it is recognized as the most common bridging therapy offered to patients on a waiting list for liver transplantation.
Variations in TACE protocols are vast. A wide range of chemotherapeutic mixtures has been reported in the literature like bland embolization, chemoembolization, and chemoperfusion. Initially, transarterial therapy for HCC was performed using bland embolization. Conventional TACE has been used for years with the gold standard mixture composed of Lipiodol mixed with chemotherapeutic agents, most commonly Doxorubicin. Recently, Drug-eluting beads are appearing as a new drug.
Drug-eluting beads have been introduced and marketed as a better chemotherapeutic carrier and embolic agent. The mechanism of DEBS action is achieved through prolongation of the contact time between chemotherapeutic agents and tumor cells with a sustained slower release of chemotherapeutic agents, which leads to lower peak plasma concentrations/systemic toxicity and theoretically greater tumor toxicity. Despite these proposed benefits of DEBS, there is limited and conflicting data on the efficacy of DEBS-TACE compared to cTACE.
The current study represents comparative clinical trial between two groups of patients, 1st group represents conventional TACE, we used (lipiodol as embolizing agent mixed with Doxorubicin), 2nd group represents DEBs-TACE, we used drug-eluting beads, Statistical analysis of our results show no significant difference between two groups regarding tumor response and liver function tests changes (baseline and 1 week after the operation).
Using drug-eluting beads can minimize complications rate, however, its cost is still limiting factor as many patients can not afford it, we recommend using DEBs-TACE in large capsulated lesions, as this can result in better tumor response.
Further multi-center randomized controlled clinical trials with larger sample sizes are recommended to address the tumor response, complications rate, liver status and changes in α-fetoprotein level to overcome current study limitations.
Conflict of interest:
The author declared that there is no conflict of interest.
Highlights/Teaching points:
- TACE is the treatment of choice for intermediate-stage HCC.
- Variations in the TACE protocol are vast. Some interventional radiologists use conventional TACE with lipiodol and Adriamycin, others use drug-eluting bead TACE with hepasphere particles.
- This clinical trial compares tumor response and liver function test changes between two groups of patients received TACE.
- We concluded that no difference in tumor response in follow up CT after 1 month, between two groups, also there is no difference in liver function tests changes in between two groups.
Abbreviations:
c TACE: conventional trans-arterial chemoembolization, DEB-TACE: drug-eluting beads trans-arterial chemoembolization, HCC: hepatocellular carcinoma, CR: complete response, PR: partial response, SD: stable disease, PD: progressive disease, LFTs: liver function tests.
REFERENCES
- Chandra S, Das A, Roy D, Bose P, Mukherjee L, Samanta J Banerjee R, Bakuli R, Jana M, Mukhopadhya D. Evaluation of methanolic extract of Clitoria ternatea Hepatoprotective and Nephroprotective Activity in Rats, Int. J. Pharm. Phytopharm. Res. 2019;9(4):30-8.
- Jemal A, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: aCancer Journal for Clinicians.2011;61(2):69-90.
- Hassan TR, Mohammed AS, El Desouky MA, Ibrahim MA, Elhefny MA. Evaluation of cellular tumor suppressor protein p53 antigen and 5-Methylcytosine (methylated DNA) as a Diagnostic Biomarker for Hepatocellular Carcinoma in Egypt. J Biochem Tech. 2019;10(3):98-104.
- European Association for Study of Liver, European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012; 48(5): 599-641.
- Bentayeb Y, Moumen Y, Boulahbal S, Chentouh S. The Protective Role of the Date Palm Pollen (Phoenix Dactilyfera) on Liver and Haematological Changes Induced by the Diethyl Phthalate. World Journal of Environmental Biosciences. 2018;7(4):90-4.
- Llovet JM, Burroughs A, Bruix J: Hepatocellular carcinoma. Lancet, 2003; 362: 1907-17.
- Bruix J, Sherman M: Management of hepatocellular carcinoma. Hepatology, 2005; 42: 1208-36.
- Llovet JM, Bruix J: Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology, 2003; 37: 429-42.
- Bargellini I, Florio F, Golfieri R, Grosso M, Lauretti DL, Cioni R. Trends in utilization of transarterial treatment for hepatocellular carcinoma: results of a survey by the Italian Society of interventional radiology. CardiovascinterventRadiol. 2014; 37(2): 438-44.
- Lewis AL, Gonzalez MV, Leppard SW, et al: Doxorubicin eluting beads: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med, 2007; 18: 1691-99.
- Li H, Wu F, Duan M, et al. Drug-eluting bead transarterial chemoembolization (TACE) vs conventional TACE in treating hepatocellular carcinoma patients with multiple conventional TACE treatments history: A comparison of efficacy and safety.Medicine (2019)98:21.
- Ferrer Puchol MD, La Parra C, Esteban E, et al. Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma.Radiologia.2011; 53(3):246-253.
- Malenstein H, Maleux G, Vandecaveye V, et al. A randomized phase II study of drug-eluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie2011;34:368-376.
- Song JE and Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol 2017 June 28;9(18): 808-8
- Kohla MAS, Abu Zeid MI, Al-Warraky M, et al. Predictors of hepatic decompensation after TACE for hepatocellular carcinoma. BMJ Open Gastro 2015;2:e000032.doi:10.1136/bmjgast-2015-000032.
