Archive \ Volume.11 2020 Issue 1
Effects of Iron Products on Decay, Tooth Microhardness, and Dental Discoloration: A Systematic Review
Imaneh Asgari 1, Samaneh Soltani 2*, Sayed Mohsen Sadeghi 3
1 Assistant professor, Department of Oral Public health, Dental Materials Research Center, Dental Research institute, School of Dentistry, Isfahan University of Medical SciencesIsfahan, Iran. 2 Postgraduate Student, Department of Periodontics, Dental Faculty, Isfahan University of Medical Sciences and Health Services, Isfahan, Iran. 3 Postgraduate Student, Department of Endodontics, Dental Faculty, Isfahan University of Medical Sciences and Health Services, Isfahan, Iran.

Abstract
Background: Various iron-based products are available that are prescribed in iron deficiency. However, inadequate intake of iron due to the discoloration of teeth and perception of beginning caries has been reported. In contrast, other findings indicate cariostatic property for iron. Regarding the dispersion of the results of studies on the effect of iron on the tooth and its importance, this systematic review, reviewed the effect of iron products on dental caries. Methods: To access the articles in this review, which includes Persian and English articles published up to December 2018, keywords were searched based on the outcome of caries, tooth microhardness and dental discoloration on national and international data basis. Both types of in vitro & in vivo studies related to our topic were selected then necessary Information was extracted from each study and appraised by proper checklists. Results: After removing unrelated articles, 34 articles were evaluated qualitatively, which included 30 RCT studies (13 related to caries, 11 related to microhardness and 10 related to discoloration). In decay group 1 study and microhardness group 2 studies received a low risk of bias score. Conclusion: Studies on iron salt combinations reinforce the cariostatic hypothesis, but about the iron drop, because of various configurations and additives cannot be certainty commented. Of course, showing that some iron drops show a cariostatic effect in the presence of a carious diet. In the field of microhardness, the presence of iron in the cariogenic diet decrease changes in microhardness but the acidic diet, we need more in vivo studies. Also about discoloration well-designed studies especially in human beings are required.
Keywords: iron, tooth, dental caries, microhardness, tooth discoloration

INTRODUCTION
The iron element has different functions in the body, out of which functionality of red blood cells, Myoglobin activity and participation in reactions of cellular respiration as well as energy production could be mentioned. Nutritional deficiency and anemia are some of the most common nutritional problems in the 21st century regardless of numerous foods rich in iron [1]. There have been presented three approaches to prevent iron deficiency as follows: to prescribe an iron supplement for at-risk groups (iron aid), to enrich certain foods with iron and to train to modify effective habits or behaviors through appearance and intensification of iron deficiency [2]. In the national health program of ministry, daily usage of one iron drop is prescribed for the children of 6 to 24 months to prevent iron deficiency [3]. In spite of the significance and cost of such a national plan, iron deficiency has been reported many times causing discoloration of deciduous teeth that parents consider this issue as the beginning of caries in teeth due to taking iron drops [4]. Having poor health care or any defect on the external surface of the teeth enamel makes the teeth prone to discoloration [5].
The major side effect of iron supplement reported in some studies is dental caries. An old study conducted by James and Parfitt (1953) is relevant research to medication and caries creation which has studied the effects of iron supplements with a PH between 1/5 - 8/56 on the teeth. In this study, the most effective factors in dental caries have been revealed as supplement acidity, frequency, and instruction of supplements as well as the child's talent [6].
In contrast, some studies indicate other results suggesting that iron has a cariostatic effect on dental decay [7]. The cariostatic effects of iron have been attributed to various factors, including reduction of Streptococcus Mutans biofilm [8] or inhibition of the bacterial enzyme glucosyltransferase by this metal ion [9]. Berlutti F stated that reduction in the saliva Fe results in increasing the accumulation of Streptococcus Mutans bacteria and plaque formation whereas the increase of saliva Fe prevents this issue [10]. Martinhon CC et al. (2006) showed that ferrous sulfate of demineralization decreases the blocks of cow's enamel and also changes the ionic biofilm of dental compounds [11]. In the study of Eshghi et al., performed on laboratory rats, the researcher suggested that by using iron supplements, there is the possibility to stop the process of decay despite certain complications such as dental color changes [12]. Another study in Shiraz on the relation between DMFT of children aged 2-5 and intake of iron aid, suggests iron aid intakes in various forms have no influence on the prevalence of dental decay in children and based on the opinion of the researcher, the decay that the parents consider as a result of iron drop intake may be related to the syndrome of nursing bottle dental decay [13]. Hussein AS (2013) studied four elements including iron, copper, manganese, and zinc in the saliva of people with decay and the controls which the result demonstrated no significant difference in the amount of iron and manganese in both groups [14].
An example of the results from the above studies illustrates that there was no consensus on this issue and given the dispersion of the study’s results in this field along with its importance in our country, a systematic overview has been implemented in this research to study the effect of iron products on teeth.
METHODS
Search Study:
At first, we Defined the review question and developing criteria for including studies instruction then searching for studies began [15]. The studies were divided into two general groups containing in-vitro and in-vivo. According to the PICO model, in the in-vitro group, the population (P) included extracted human or animal tooth out of the mouth. In the in-vivo study, the population contained the human and animal teeth in the oral cavity. The understudy intervention (I) was to expose the teeth to iron drop, iron supplement or other iron products. Comparing (C) with the teeth which weren’t exposed to iron drop, iron supplement and in general iron products. The understudy outcomes of (O) included dental hardness, dental decay, and color change.
The keywords concerning English and their equivalents in Farsi were searched through national databases (SID, Iranmedex) and international databases (Medline (base PubMed), Scopus and Google scholar) (Figure 2). The references of the found papers were used to have access to the relevant papers. The hand searching was carried out through dissertations of Isfahan University of Medical Sciences and the Islamic Azad University of Khorasgan.
Study Selection Criteria
The inclusion criteria of this systematic review has covered all interventional and/or observational studies on the effect of iron products on human and animal teeth focusing on caries, color changes and microhardness of tooth in oral environment(In-vivo), or in laboratory environments (In-vitro) that have been published in Persian (local language) or English by October 2018 and their abstracts are accessible in online databases.
The studies in the in-vivo subgroup using a non-edible iron form with no exposure to the teeth were excluded. Moreover, the removed studies included studies that had examined the effect of the iron element along with fluoride on teeth or pregnant mothers, as well as the impact of iron products indirectly on the infants.
After the initial search and Identifying multiple reports from the same study, firstly the title and abstract of the article were studied independently by two researchers to remove irrelevant reports and the full text of the potentially relevant reports was studied afterward. Any disagreement between the two researchers was Corresponded with investigators, to clarify study eligibility and final decisions on study inclusion and proceed to data collection was made.
Data Extraction & Assessment of Risk of Bias of the Studies:
After the selection of qualified articles, the necessary information was extracted separately by the two researchers. The recorded data of each study including the name of the first author, the year of publication, the type of study, the summary of methodology (intervention, sampling method, data collection method) and the results were used to prepare the Evidence tables.
To review and criticize the eligible studies, the relevant checklist based on the study design was used. To study RCT (including Randomized clinical trials and Randomized control trials, the checklist submitted in the handbook of the Cochrane version (5.1.0) was used. The number 1 and 0 were given to every low-risk of bias and high-risk of bias cases, respectively. The cut point of 5 was put the study in the category of low-risk of bias, 3-4 for moderate-risk of bias and less than 3 for high-risk of bias. To criticize case-control and cohort, the Newcastle-Ottawa checklist [16] was utilized and the borderline between high quality and low quality was number 6. CASP checklist was used for cross-sectional articles in which scoring agreement between the two researchers was as follows: 8 and higher for high quality, 4-7 for moderate quality and lower than 4 for low quality. According to the agreement of the researchers, RCT articles with qualitative scores of equal or lower than 1or other articles with a score equal to or lower than 2 were supposed to be excluded.
Two authors independently assessed the risk of bias of the included articles. Any disagreements between the authors were resolved by consensus and/or consultation by a third person. the level of agreement between the two review authors was evaluated with the Cohen kappa statistic which was 86%.
Due to the heterogenization of the results related to the studies, it was not possible to conduct statistical analysis in this study.
The search study is shown in figure 2.
RESULTS:
Presenting Results and ‘Summary of findings’ Tables:
The outcome of electronic and manual searches was 2495 articles through which 2425 unrelated articles were removed after studying the title and abstract. After the removal of 21 duplicated articles out of 70, 49 studied articles remained. Three articles, Jokinen MA [17], Muszyńska-Zimna E [18] and Lokken P [19] were removed due to their language so just the abstracts were studied. Access to the abstract and full text of Parkinson CF [20], Lacy AM [21], and Torell P [22] articles was not provided. Also, the full article of Xavier AM, Sintes JL and Torell P [23-25] were not accessible. 6 other articles were eliminated due to inclusion & exclusion criteria’s and finally, 34 articles were studied qualitatively.
Outcome 1: tooth decay, In the study of dental caries studies, 13 studies were conducted in which 10 of them were RCT and two of them were case-control studies and one case was an interventional study without a control group. The randomized controlled trial included 4 in-vitro studies and 6 in-vivo animal studies performed on hamsters and laboratory rats.
The iron drop has been used as a test product in five studies of the interventional studies, out of which four studies were in-vitro (two high risks of bias studies and two moderate-risk of bias studies) and one study was in-vivo (moderate-risk of bias) on the rats. Two case-control studies also investigated the relationship between decay and history of iron drop intake. In other studies, iron salts compounds had been used.
In-vivo studies: A study with a low risk of bias supports the reduction of decay in laboratory rats by the use of ferrous sulfate and sucrose [26]. Three studies with a moderate risk of bias support decay reduction in the presence of iron or iron drop with sucrose in the diet of laboratory rats [12, 27, 28]. However, such a decrease in the decay of rats was not observed by adding an iron drop to a non-cariogenic diet [12]. Two other studies with a high risk of bias also support the reduction in the incidence of decay by iron intake in the diet of rats [29, 30].
In-vitro studies: The results of two studies with a moderate risk of bias suggested that iron drops depending on the brand and their compounds along with sucrose, can show cariostatic effects themselves or not [7, 31].
The result of a high–risk of bias study also indicates a decay decrease in the case of an iron drop in addition to the cariogenic environment [32]. Another study with the same quality stated that adding iron concludes the reduction of losing tooth minerals [33].
Parfitt's study also indicated that permanent exposure of the teeth with more acidic iron drops leads to more damage to the teeth surface [6].
Two case-control studies with low-quality grade stated that the history of iron drop intake had no relationship with the prevalence of decay in children between the ages range of 1-5 years [13, 34]. Results are shown in table 1, 4 ,5 & 10.
Outcome 2: microhardness, In the field of microhardness, eleven kinds of research were studied, some of which used cariogenic environments attempting to renovate cariogenic conditions by applying a series of acidic environments to reconstruct the dental erosion environment. All found RCT studies (randomized control trial and randomized clinical trial) were randomized among which there were three in-vivo studies and the rest were in-vitro. Among them, two studies of moderate risk of bias had been conducted using iron drops in-vitro and the rest had utilized iron salts.
Investigating studies in decay simulated condition: Two in-vivo studies with the low-risk quality specified that in the human oral environment, the association of iron with sucrose has caused a reduction in the microhardness changes of the cows or human enamel blocks [8, 11]. An in-vitro study with a moderate-risk of bias showed that changes in microhardness decrease by increasing iron concentration along with sucrose [35]. Another in-vitro study with the same qualitative grade, by adding an iron drop to a cariogenic environment demonstrated that iron drop does not lead to reducing microhardness changes [36].
Investigating studies in erosion simulated: Four studies with a moderate risk of bias declare that adding iron to acidic drinks and the presence of iron in the acidic environment leads to a decrease in demineralization in the in-vitro [37-39] and in-vivo environment in the human mouth [40].
An in-vitro study with a moderate risk of bias suggests iron in the presence of an acidic drink containing citric acid does not have an inhibitory effect on preventing demineralization, however, if the acidic drink contains phosphoric acid, such effect is expected from iron [41].
A study with a moderate risk of bias stated that adding iron to acidic drinks increases dental microhardness changes in in-vitro [42]. Another study with the same degree of quality using iron drop suggested exposure of the teeth with an iron drop in in-vitro ends up with a decline in dental hardness [43]. Results are shown in table 2, 6, 7 & 11.
Outcome 3: tooth color change: Regarding this outcome, 10 articles were studied including 1 cross-sectional study and 9 RCTs out of which there were 6 in-vitro and 3 in-vivo studies. In total, 5 in-vitro studies with a moderate risk of bias have covered this effect of iron drop.
In-vivo studies: In the moderate-risk of bias study, the iron intake in the form of the tablet did not lead to color change [44]. Another moderate risk of bias study considers color change as a result of iron intake related to diet [45]. A high risk of bias study by sampling dental pigments with graphite curette showed that metal ions are not seen in this color change [46].
In-vitro studies: Three moderate-risks of bias studies investigating the effect of iron drop stated that the intensity of color change as the result of iron drops varies among different brands [47]. The dental color change is more severe in a cariogenic environment [48]. And iron absorption on the tooth surface also increases following the surface etching [49]. The combination of the drop containing ferric oxide polymaltose and Ferro-fumarate creates less color change comparing to any products individually [50]. Another moderate risk of bias study declares that an increase in iron concentration is related to the increase of dental color change [51]. One more moderate-risk of bias study indicated that the mean of iron atomic absorption by teeth among different iron drops being studied did not have any significant statistical difference [52].
In a low-quality cross-sectional study, the prevalence of tooth discoloration was higher in children with a history of iron drop intake [53]. Results are shown in table 3, 8, 9 & 12.
DISCUSSION:
The actual review study was devised and conducted systematically to determine the effects of various iron compounds on the teeth considering the wide available variables. Regarding decay, although studies on iron ions and iron salts compounds reinforce its cariostatic hypothesis, it is not probable to comment definitely on iron drop due to a variety of compounds and additives. However, it seems that certain iron drops show a cariostatic effect in the presence of a cariogenic diet [12, 31]. About the present information, it is probably possible to mention that in case a child takes sugars to be used by Cariogenic bacteria, the cariostatic effect could be regarded based on iron drop intake (especially the form of ferrous sulfate, which has been effective in most researches to date). In the field of microhardness, the presence of iron along with sucrose has led to a reduction in microhardness changes of cows and humans enamel. Furthermore, the addition of iron to acidic drinks causes a decrease in demineralization in in-vitro [37-39] and in-vivo [40] environments. There is still disagreement over the effective mechanism of such ion and its various forms, and there is a fascinating research field in this regard. Concerning the effect of iron in reduction of surface changes (SMH %), it seems that enamel in an acidic diet similar to the clinical status of erosion (such as studies of Buzalaf [38], Kato [41], Xavier [39], etc.), as well as the effect of this ion due to presence of cariogenic diet, play a role in the mechanism of iron effectiveness, which is an extra process on the effect on cariogenic bacteria. To date, to justify this issue, it is said that the incubation of Ferro sulfate to enamel leads to the deposition of ferric phosphate and the formation of an acid-resistant layer on the enamel surface [24]. It has been recommended to record this ferric phosphate in higher microhardness can be explained in two different ways as follow: by preventing the exposure of the tooth surface to an acidic drink resulting in reduction of the demineralization, or the indenter of the microhardness meter is not only in contact with apatite crystals, but also it is in contact with ferric phosphate ions causing higher microhardness recording [54]. Further studies are required regarding this matter in the in-vivo environment and humans.
It should be mentioned that the studies conducted on iron drops with acidic PH in an in-vitro environment do not apply to the in-vivo environment due to the salivary properties of oral saliva.
No article about a low risk of bias in color change was found in kinds of literature so the results were not coherent. It appears that consuming foods rich in iron (such as eggs, vegetables, etc.) results in the growth of certain bacteria that cause black pigmentation in the teeth [53]. It has been exhibited that the saliva of the children in which black-pigmentations exist, contains more calcium and phosphate, therefore it can increase the buffering properties of the saliva and be the cause of a decrease in the prevalence of decay in the presence of pigmentation [55, 56]. However, the contrast existing among diet, pigmentation, decay and oral flora has not been identified yet. The coloring feature of the iron drop is more attributed to the combination of iron with sulfide ions as a result of the bacteria activity. The color change varies through different iron drop intake which could be related to the whole amount of iron available in each drop, the acidity and the ability to etch the tooth surface by each drop, any individual’s diet, bacterial flora, and so on to justify no sign of color change in all consumers. To sum up, the hypothesis as follows could be deducted by reviewing in-vitro studies that intake of iron drop as well as exposure to the teeth due to acid content could lead to a status similar to etching teeth and following the increase of prosthetics, iron absorption will be enhanced.
The presence of early decays also has a similar role as increasing prosthetics. There is the possibility of an iron sulfide compound to be formed in the presence of ion sulfide, and in case it reaches a proper concentration, the clinical color change could be seen. This sulfide is caused by the activity of bacteria. It could be possible that by eliminating other bacteria iron leads to the predominance of these sulfide-producing bacteria. It has been illustrated that iron could have an inhibitory and fatal effect on some groups of bacteria with an unknown mechanism. It is likely to raise such a hypothesis that this mechanism can cause a cariostatic effect of iron and predominance of sulfur-producing bacteria and, consequently, the possible increase in the creation of dental pigmentation. In case of intensification in iron ion penetration, for any reason including more prosthetics of teeth surface as the result of primitive decay or acidity of an iron drop, frequency of intake and so on, it is difficult to remove the color.
In-vivo studies in humans require further study because the two studies did not provide the results confirming the above-mentioned hypotheses: 1) Martinhon's research studying iron amount in dental plaque and enamel did not observe any increase in the iron amount in enamel in spite of dental plaque [11]. 2) Also, in sampling the Parnas study by the use of graphite curette from the black-colored pigment of a tooth, no metal ions were seen [46].
An example of this study limitations include the lack of access to the full text of all articles and English and Persian version of certain articles was not available despite consisting of invaluable information, therefore it was no possibility to use them.
CONCLUSION:
The study findings demonstrated that the association of iron and iron drop with sucrose causes a reduction in the prevalence of decay. However, regarding accompaniment of other diets with iron further studies are required, especially in the in-vivo environment. The mechanism of cariogenic effect of iron has not been proved yet to date. About the effect of adding iron to acidic drinks through reducing microhardness more in-vivo and in-vitro studies are needed. Following the iron drop intake, the color change is expected but it is not obvious whether it is merely an independent outcome or it has a relation with iron cariostatic mechanism. It is to say that the best way to prevent dental color changes is to avoid exposing the child’s teeth with the iron drop due to not having enough information on unknown complicated mechanism of the iron effects. The obvious issue is that the color changes are not considered as decay although it is possible some initial decays have been provided the basis for the color change through the creation of prosthetics. It is maybe possible to say the presence of the color change could be as a result of decreasing decay activity in the oral cavity; however, it is quite an elementary hypothesis which requires further research.
It is suggested to use extensive cohort studies in addition to further research on recognizing the mechanisms of iron effect, considering the difficulty of controlling and simulating the oral environment as well as the multiplicity of independent and confounding variables. Moreover, it may be possible to reduce the directive target population of the iron drop in a country by using other enriching methods.
Funding:
This study has been supported by Isfahan University of Medical Sciences.
Acknowledgments:
Based on a thesis submitted to the School of Dentistry, Isfahan University of Medical sciences, in partial fulfillment of the requirement for the DDS/MSC degree. This study was supported by Isfahan University of Medical Sciences Research Grant#395577
Competing Interests:
The authors of this manuscript declare that they have no conflicts of interest, real or perceived, financial or nonfinancial in this article.
|
Table 1. Relevant studies to tooth decay |
||
|
First author |
Year |
Study type |
|
Mcclure [30] |
1948 |
In-vivo RCT |
|
Parfitt [6] |
1953 |
In-vitro Non-random intervention |
|
Miguel [26] |
1996 |
In-vivo RCT |
|
Rosalen [28] |
1996 |
In-vivo RCT |
|
Miguel [27] |
1997 |
In-vivo RCT |
|
Emilson [29] |
1972 |
In-vivo RCT |
|
Weiss [33] |
1985 |
In-vitro RCT |
|
Naderi [34] |
2002 |
Case-control |
|
Eskandarian [13] |
2005 |
Cross-sectional |
|
Thakib [7] |
2006 |
In-vitro RCT |
|
Thakib [31] |
2009 |
In-vitro RCT |
|
Eshghi [12] |
2012 |
In-vivo RCT |
|
Esmaeilzadeh [32] |
2015 |
In-vitro RCT |
|
Table 2. relevant studies to dental microhardness |
||
|
Author |
year |
Study type |
|
Martinhon [11] |
2005 |
In-vivo RCT |
|
Pecharki [8] |
2005 |
In-vivo RCT |
|
Kato [41] |
2006 |
In-vitro RCT |
|
Sales-Peres [40] |
2006 |
In-vivo RCT |
|
Buzalaf [38] |
2006 |
In-vitro RCT |
|
Kato [42] |
2007 |
In-vitro RCT |
|
Eskandarian [36] |
2013 |
In-vitro RCT |
|
Karina [37] |
2011 |
In-vitro RCT |
|
Cláudia [35] |
2012 |
In-vitro RCT |
|
Pasdar [43] |
2015 |
In-vitro RCT |
|
Xavier [39] |
2015 |
In-vitro RCT |
|
Table 1. relevant studies to tooth discoloration |
||
|
Author |
year |
Study type |
|
Lokken [44] |
1979 |
In-vivo RCT |
|
Addy [45] |
1985 |
In-vivo RCT |
|
Stangel [51] |
1996 |
In-vitro RCT |
|
Shabzendehdar [49] |
2006 |
In-vitro RCT |
|
Mehran [48] |
2008 |
In-vitro RCT |
|
Shojaipour [47] |
2009 |
In-vitro RCT |
|
Limor parnas [46]
|
2013 |
In-vivo RCT |
|
Garcia Martin [53] |
2013 |
Cross sectional |
|
Pani [50] |
2015 |
In-vitro RCT |
|
Hekmatfar [52] |
2018 |
In-vitro RCT |
|
Table 2. In-Vivo Studies relevant to tooth decay |
|||||
|
First author |
Population & sample size |
Form(s) of iron & intervention |
Control group |
Follow up |
Outcome- measurement |
|
.Mcclure [30] |
Rats
|
Chloride citrate |
Diet without iron ions |
N.M |
Iron is not only noncariogenic but also reduced caries prevalence |
|
Miguel [26] |
Rats N=12 |
sucrose containing FeSO4
|
Plain sucrose |
3 weeks |
tooth caries & microbial counts |
|
Rosalen [28] |
Rats N=96 |
adding Fe to sucrose
|
Plain sucrose |
21 days |
Caries scores & microbial assessment |
|
Miguel [27] |
Rats N=48 |
Adding FeSO4, & ferric glycerophosphate to sucrose
|
Plain sucrose
|
3 weeks |
Dental decay dental color change, microbial count & plaque PH changes |
|
Emilson [29] |
Hamsters N=Approximately 30 N.M Exactly |
1st: adding FeCl3 to water & diet 2nd: Appling FeCl3 & FeSo4 topically on rat’s teeth 3rd: adding FeSo4 to water and rat’s diet
|
A group without iron in each experiment |
13 weeks
|
Dental decay; the amount of Strep. mutans in biofilm; Plaque formation |
|
Eshghi [12] |
Rats N=12 |
Cariogenic diet + iron supplement & Noncariogenic diet + iron supplement |
Cariogenic & Noncariogenic diet without iron
|
4 months |
progression of caries & the depth of the lesions in the enamel in histologic samples |
|
Table 5. In Vitro studies relevant to tooth decay |
|||||
|
First author |
Population & sample size |
Form(s) of iron & intervention |
Control group |
Follow up |
Outcome- measurement |
|
Parfitt [6] |
Extracted sound teeth N= NM |
5 types of an iron drop with different acidity |
N.M |
A week |
Damage to the teeth surface (Visual) |
|
Weiss [33] |
Extracted sound teeth N=10 |
FeCl3 was used as a supplement to acid gel, or as pretreatment of the enamel surfaces before exposure to the pure gel.
|
Groups without using iron salts |
16 weeks |
Differences in light transmission of groups from the radiographs taken before and after
|
|
Thakib [7] |
healthy premolar teeth N=60 |
adding iron to test tubes with bacteria & sucrose |
Test tubes with only sucrose & bacteria |
29 days |
Assessment of decalcification and cavitation |
|
Thakib [31] |
healthy premolar teeth N=200 |
4 types of iron supplements plus strep. mutans |
Positive control (without iron)& negative control (without iron & bacteria) |
60 days |
Assessment of decalcification and cavitation |
|
Esmaeilzadeh [32] |
Sound extracted primary teeth N=128 |
Adding an iron supplement to artificial decay-causing environment |
Only artificial decay-causing environment
|
60 days
|
Decalcification and its progress
|
|
Table 3. In vivo studies relevant to tooth microhardness |
|||||
|
First author |
Population & sample size |
Form(s) of iron & intervention |
Control group |
Follow up |
Outcome- measurement |
|
Martinhon [11] |
Bovine enamel blocks used by 12 volunteers
|
Using a solution of ferrous sulfate followed by a sucrose solution
|
sucrose solution without iron pretreatment |
14 days |
the percentage of surface microhardness change - ferrous sulphate reduced the demineralization of enamel block |
|
Pecharki [8] |
Blocks of human enamel Used by 16 volunteers
|
20% (w/v) sucrose plus 18 microg Fe/ml, and 20% (w/v) sucrose plus 70 microg Fe/ml |
1) water; 2) 20% sucrose |
14 days |
Analyzing biofilms formed on the blocks, determining mineral loss of enamel- Lower demineralization was found in the test group |
|
ales-Peres [40] |
Enamel & dentin blocks of human extracted third molar used by 10 volunteers
|
volunteers immerse appliances in cola drink then rinsed with Ferrous sulfate solution Then one group brushed immediately & another one after 30 minutes
|
The same procedure without using an iron solution |
5 days |
Changes in surface microhardness and wear of enamel and dentine - The iron solution caused a significant reduction on the %SMH in enamel and a significant reduction on the wear in dentine |
|
Table 7. In Vitro studies relevant to tooth microhardness |
|||||
|
First author |
Population & sample size |
Form(s) of iron & intervention |
Control group |
Follow up |
Outcome- measurement |
|
Melissa [41] |
Enamel powder from bovine incisor
|
the carbonated beverage containing different concentrations (1.25, 2.50, 5, 10, 15, 30 and 60 mmol/l) of FeSo4 |
carbonated beverage with no iron |
centrifuged: 30 s supernatant was removed at 1 min 40 s.
|
The phosphate released in the medium - iron can interfere with the dissolution of dental enamel powder in the presence of acidic beverages which influence by the type of acid |
|
Buzalaf [38]
|
Powdered enamel from bovine incisor fragments |
Part1: Enamel powder was subjected to acetic acid made with increasing concentrations for FeSO4_7H2O part2: Enamel blocks were exposed to a sequence of seven plastic vials containing acetic acid. Also, iron added in in the fourth vial
|
In part 2: In the control group, there wasn’t iron in 4th vial |
- |
phosphate released in the medium was analyzed – Fe(2+) can be effective on inhibition of the dissolution of enamel and that this effect may be durable. |
|
Kato [42] |
blocks of bovine enamel N=48 |
Blocks exposed to cycles of demineralization in Coke containing 10 mmol/L of iron then cycles of remineralization in artificial saliva |
Blocks exposed to cycles of demineralization in Coke Without iron and then cycles of remineralization in artificial saliva |
4 cycles in each process |
% superficial microhardness change (SMHC) and wear analysis- iron at 10 mmol/L significantly reduced the wear but significantly enhanced the %SMHC of enamel blocks submitted to erosion by Coke. |
|
Eskandarian [36] |
Anterior primary sound teeth N=90 |
Teeth divided into 4 groups and were placed in a cariogenic medium with 4 types of iron drop |
1)cariogenic medium without iron 2)culture medium without S. mutans |
14 days |
Enamel & dentin microhardness iron supplementation did not affect the demineralization of tooth structure. |
|
Karina [37] |
blocks of bovine enamel N=110 |
50 blocks were subjected to demineralization pH cycling & additional iron treatment (FeSO4_7H2O) 50 blocks with artificially demineralized lesions subjected to remineralization pH cycling & additional iron treatment |
Groups with no iron treatment in demineralization & remineralization cycles |
Demineralization cycle = 7 days Remineralization cycle = 6 days |
surface hardness, fluoride, calcium, phosphorus, and iron of enamel blocks- iron reduces demineralization but does not allow remineralization to occur. |
|
Cecília [35] |
blocks of bovine enamel N=44 |
Biofilms on blocks were exposed to sucrose, and then subjected to FeSo4 (3 different concentration)
|
NaCl as a negative control, Chlorhexidine (CHX) as a positive antibacterial control; NaF as a positive anticaries control |
6 days |
percentage of SH loss (%SHL) Biofilm composition (viable bacteria, EPS formation,)– Iron treatment reduced the number of viable bacteria formed in the biofilm and also reduced the enamel’s %SHL
|
|
Nilgoon [43] |
Anterior primary teeth N=40 |
Teeth were subjected To 4 types of iron drop
|
N.M |
5 minute in a solution |
surface microhardness - In all groups, microhardness was decreased but it was more in a group with the most acidic drop.
|
|
Xavier [39] |
enamel blocks (primary and permanent teeth) n=180 |
beverages supplemented with iron (FeSO4.7H2O)
|
Not adding iron to beverages |
3 treatment cycles of 5/20 minute incubation periods |
calcium and phosphate released after each cycle, the final SMH - Adding iron supplementation to acidic beverages is beneficial in reducing mineral loss |
|
Table 4. In vivo studies relevant to tooth discoloration |
|||||
|
First author |
Population & sample size |
Form(s) of iron & intervention |
Control group |
Follow up |
Outcome- measurement |
|
Lokken [44] |
Young women N=19 Cross-over study |
ferrous fumarate tablets (Neo-Fer*) |
placebo |
8 weeks |
Plaque formation, demineralization, and discoloration No tendency of this oral iron to cause dental discolorations
|
|
Addy [45] |
5 Adults |
CHX- CHX& tea- CHX v Iron- Iron- Iron & tea- tea |
Cross-over study with 2 days interval |
1 day |
Dental & tongue discoloration- tooth discoloration due to iron is also related to diet |
|
Limor [46]
|
Children N=26 |
Collecting dental plaque from 17 children with black stain using graphite curette And a metal curette for 4 children with stain |
Collecting dental plaque from 15 children without stain with graphite curette |
- |
Metallic ions do not seem to be the origin of dental black stain |
|
Table 5. In Vitro studies relevant to tooth discoloration |
|||||
|
First author |
Population & sample size |
Form(s) of iron & intervention |
Control group |
Follow up |
Outcome- measurement |
|
Stangel [51] |
Extracted human teeth N=N.M
|
iron solutions (ferric oxalate-nitric acid solutions, or citric acid-ferric chloride solution) applied to the surface of the sample. after rinsing with water submersed in the sodium-sulfide solutions |
Not preparation with iron |
60 S in iron solution 18 H in NaS solution |
it seems that increasing iron concentration related to discoloration XPS detected iron in enamel but not in dentin, while EDS detected iron in both enamel and dentin |
|
Shabzendehdar [49] |
anterior primary teeth n=60 |
Etched enamel treated with iron supplements |
Non-etched enamel treated with iron supplements |
- |
There was a difference in the iron absorption between iron drops The absorption in etched teeth increased |
|
Mehran [48] |
Primary teeth N=93 |
2 groups of teeth were immersed in artificial caries challenge (ACC) before exposure to iron drop
|
2 groups of teeth were immersed in normal saline before exposure to iron drop
|
- |
Being immersed into the ACC caused more iron absorption, severe discoloration & structural changes in the enamel of primary teeth which is more obvious in one of the drops than the other |
|
Shojaipour [47] |
primary incisor teeth n=60 |
teeth in each stage were divided into three groups and each group was exposed to one kind of Iron drop |
- |
2 phase with a one-week interval |
Although all three Iron drops cause stain on primary incisor teeth, there is a significant difference among them |
|
Pani [50] |
primary central incisors N=40 |
G1: ferric oxide polymaltose G2: ferrous fumarate G3: combination of iron compounds |
Only artificial saliva |
72H |
combining different forms of iron seems to elicit a lower intensity of staining than equivalent doses of a single form of iron. |
|
Hekmatfar [52] |
anterior primary teeth N=40 |
Teeth divided into 5 groups then exposed to 5 kinds of iron drop
|
|
|
There was no significant difference between the mean iron ion adsorption, as well as between iron ion absorption with pH and TA also not found a statistically significant relationship. |
|
Table 6. other studies relevant to tooth decay |
||||
|
First author |
Study type |
Population & sample size |
Form(s) of iron & intervention |
conclusion |
|
Naderi [34] |
Case-control |
children aged 1-4 years N=60 |
using iron supplements |
There was no significant difference in the mean of DMFT between case and control group. |
|
Eskandarian [13] |
Cross-sectional |
children aged 2-5 years N=280 |
using iron supplements |
There was no significant difference for the mean of DMFT between case and control group
|
|
Sintes [23] |
No full-text access The abstract states that adding iron to the diet leads to a reversal of the process of dental caries and reduces its prevalence |
|||
|
Table 7.other studies relevant to tooth microhardness |
||||
|
First author |
Study type |
Population & sample size |
Form(s) of iron & intervention |
conclusion |
|
Torell [24] |
No full-text access Iron can produce an acid-resistant layer on the enamel surface. |
|||
|
Xavier [25] |
No full-text access Ferrous fumarate had lower demineralization in compare to ferrous sulfate and more decrease in microhardness changes |
|||
|
Table 12. other studies relevant to tooth discoloration |
||||
|
First author |
Study type |
Population & sample size |
Form(s) of iron & intervention |
conclusion |
|
Garcia [53] |
Cross-sectional |
3272 children aged 6 years old |
determine the prevalence of black stain and associated risk factors in Spanish preschool children |
The regular consumption of foods rich in iron and the use of iron supplements during pregnancy and early childhood could favor the development of chromogenic microbiota |
REFERENCES
- Mahan L, Escott Stump S, Raymond J. Krause's Food & the Nutrition Care Process,(Krause's Food & Nutrition Therapy). Philadelphia: WB Saunders. Elsevier; 2012.
- Geissler, Catherine, and Hilary Powers. Fundamentals of Human Nutrition E-Book: for Students and Practitioners in the Health Sciences. Elsevier Health Sciences, 2009.
- Circularof children supplements, HealthDeputy. Ministery of Health, Treatment and MedicalEducation. Available at: [Available from: http://health.mazums.ac.ir/dorsapax/userfiles/file/behdasht/ mokamelha.doc.
- Mozaffari, Khosravi H., M. Hosseinzadeh, And Khosravi V. Mozaffari. "The study of Iron-drop supplementation status on 6-24 month infants in Yazd health centers." 2010: 56-66.
- Sulieman, Munther. "An overview of tooth discoloration: extrinsic, intrinsic and internalized stains." Dental update 2005; 32(8): 463-471.
- Parfitt, G. J. "Local effects of certain medicaments on the teeth." British medical journal 1953; 2(4848): 1252.
- Al-Shalan, Thakib A., and Amal Al-Askar. "In vitro effect of different concentrations of iron on the initiation of dental caries: pilot study." Saudi Dent J 2006; 18(2): 86-90.
- Pecharki, G. D., J. A. Cury, AF Paes Leme, C. P. M. Tabchoury, AA Del Bel Cury, P. L. Rosalen, and W. H. Bowen. "Effect of sucrose containing iron (II) on dental biofilm and enamel demineralization in situ." Caries research 2005; 39(2): 123-129.
- Devulapalle, K. S., and G. Mooser. "Glucosyltransferase inactivation reduces dental caries." Journal of dental research 2001; 80(2): 466-46
- Francesca, Berlutti, Maria Ajello, Pietro Bosso, Clara Morea, Petrucca Andrea, Antonini Giovanni, and Valenti Piera. "Both lactoferrin and iron influence aggregation and biofilm formation in Streptococcus mutans." Biometals 2004; 17(3): 271-278.
- Martinhon CCR, de Moraes Italiani F, de Magalhães Padilha P, Bijella MFTB, Delbem ACB, Buzalaf MAR. Effect of iron on bovine enamel and on the composition of the dental biofilm formed “in situ”. Archives of oral biology. 2006; 51(6):471-5.
- Eshghi, A. R., R. Kowsari-Isfahan, M. Rezaiefar, M. Razavi, and S. Zeighami. "Effect of iron containing supplements on rats’ dental caries progression." Journal of dentistry (Tehran, Iran) 2012; 9(1): 14.
- Eskandarian, T., and M. J. Joshan. "Evaluation of the DMFT index and its relationship to some factors consisting the consumption of iron supplementary drugs in 2-5 years old Kindergarden children in Shiraz." Journal of Dentistry 2006; 6(3, 4): 1-9.
- Hussein, A. S., H. F. Ghasheer, N. M. Ramli, R. J. Schroth, and M. I. Abu-Hassan. "Salivary trace elements in relation to dental caries in a group of multi-ethnic schoolchildren in Shah Alam, Malaysia." European journal of paediatric dentistry: official journal of European Academy of Paediatric Dentistry 2013; 14(2): 113-118.
- Higgins, J. P. T., and S. R. Green. "Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0; 2011." 2008.
- Wells, George A., P. Tugwell, Dianne O’Connell, V. Welch, J. Peterson, B. Shea, and M. Losos. "The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses." 2015.
- Jokinen, M. A. "The harmful effect of liquid iron compounds on human teeth in vitro." Suomen Hammaslaakariseuran toimituksia= Finska tandlakarsallskapets forhandlingar 1968; 64(3): 76-79.
- Muszyńska-Zimna, E., and G. Jerzyńska-Ponomarenko. "Effect of an iron-enriched diet on the condition of the mouth and teeth in children 1-4 years of age." Czasopismo stomatologiczne 1984; 37(11): 853.
- Lokken, P. "Do iron preparations damage the teeth?." Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke 1975; 95(10): 610.
- Parkinson, C. F., and S. B. Parkinson. "Effect of vitamin-iron preparation on tooth staining studied." Journal of the Missouri Dental Association 1981; 61(4): 34.
- Lacy, A. M. "The effect of iron compounds on dental caries: a review." Clinical preventive dentistry 1979; 1(3): 6-7.
- Torell, Per. "Iron and dental hard tissues; experimental studies concerning the significance of iron in the physiology and pathology of human enamel and dentine." Odontologisk tidskrift 1955; 63(2): 131-172.
- Sintes, J. L., and S. A. Miller. "Influence of dietary iron on the dental caries incidence and growth of rats fed an experimental diet." Archivos latinoamericanos de nutricion 33, no. 2 (1983): 322-338.
- Torell, Per. "Iron and dental caries." Swedish dental journal 1988; 12(3): 113-1
- Xavier, Arun M., Kavita Rai, Amitha M. Hegde, and Suchetha Shetty. "A spectroscopic and surface microhardness study of enamel exposed to beverages supplemented with ferrous fumarate and ferrous sulfate. A randomized in vitro trial." American journal of dentistry 2016; 29(3): 132-136.
- Miguel, J. C., W. H. Bowen, and S. K. Pearson. "Effects of frequency of exposure to iron-sucrose on the incidence of dental caries in desalivated rats." Caries research 1997; 31(3): 238-243.
- Miguel, J. C., W. H. Bowen, and S. K. Pearson. "Effects of iron salts in sucrose on dental caries and plaque in rats." Archives of oral biology 1997; 42(5): 377-383.
- Rosalen, P. L., S. K. Pearson, and W. H. Bowen. "Effects of copper, iron and fluoride co-crystallized with sugar on caries development and acid formation in desalivated rats." Archives of oral biology 1996; 41(11): 1003-1010.
- Emilson, C. G., and B. Krasse. "The effect of iron salts on experimental dental caries in the hamster." Archives of oral biology 1972; 17(10): 1439-1443.
- McClure, F. J. "Observations on Induced Caries in Rats: VI. Summary Results of Various Modifications of Food and Drinking Water." Journal of dental research 1948; 27(1): 34-40.
- Al-Shalan, Thakib A. "In vitro cariostatic effects of various iron supplements on the initiation of dental caries." The Saudi dental journal 2009; 21(3): 117-122.
- Esmaeilzadeh M, Mojarad F, Donyavi Z, Mashouf RY, Sarijeh NK. Using iron supplements for prevention of dental caries: An experimental study. Avicenna Journal of Clinical Microbiology and Infection. 2015;2(3).
- Weiss, G., A. Stabholz, A. Markitziu, I. Meyer, L. Brayer, and I. Gedalia. "The effect of iron on in vitro decalcification of human tooth enamel." Journal of oral rehabilitation 1985; 12(1): 91-93.
- I N. Naderi I. Clinical evaluation of the effects of iron drop on decididous teeth caries of 1-4 years old childeren reffering to Isfahan Saint Navab Safavi Theraputic Health Center. 2002.
- Ribeiro, Cecília Cláudia Costa, Renzo Alberto Ccahuana-Vásquez, Cadidja Dayane Sousa do Carmo, Cláudia Maria Coêlho Alves, Tarcísio Jorge Leitão, Lisandra Rocha Vidotti, and Jaime Aparecido Cury. "The effect of iron on Streptococcus mutans biofilm and on enamel demineralization." Brazilian Oral Research 2012; 26(4): 300-305.
- Eskandarian, Tahereh, Mohammad Motamedifar, Somayeh Hekmatfar, and Ali Mohammad Tamaddon. "Comparison of the effect of three types of iron drops on surface roughness of deciduous teeth in a simulated cariogenic environment." Shahid Beheshti University Dental Journal 2013; 31(1): 15-22.
- Alves, Karina Mirela Ribeiro Pinto, Karina Simões Franco, Kikue Takebayashi Sassaki, Marília Afonso Rabelo Buzalaf, and Alberto Carlos Botazzo Delbem. "Effect of iron on enamel demineralization and remineralization in vitro." Archives of oral biology 2011; 56(11): 1192-1198.
- Buzalaf, Marília Afonso Rabelo, Flávia de Moraes Italiani, Melissa Thiemi Kato, Cleide Cristina Rodrigues Martinhon, and Ana Carolina Magalhães. "Effect of iron on inhibition of acid demineralisation of bovine dental enamel in vitro." Archives of oral biology 2006; 51(10): 844-848.
- Xavier, Arun M., Kavita Rai, Amitha M. Hegde, and Suchetha Shetty. "A spectroscopic and surface microhardness study on enamel exposed to beverages supplemented with lower iron concentrations." Journal of Clinical Pediatric Dentistry 2015; 39(2): 161-167.
- Sales-Peres, S. H. C., Juliano Pelim Pessan, and M. A. R. Buzalaf. "Effect of an iron mouthrinse on enamel and dentine erosion subjected or not to abrasion: an in situ/ex vivo study." archives of oral biology 2007; 52(2): 128-132.
- Kato, Melissa Thiemi, Andrea Gutierrez Maria, Sílvia Helena de Carvalho Sales-Peres, and Marília Afonso Rabelo Buzalaf. "Effect of iron on the dissolution of bovine enamel powder in vitro by carbonated beverages." Archives of oral biology 2007; 52(7): 614-617.
- Kato, Melissa Thiemi, Sílvia Helena de Carvalho Sales-Peres, and Marília Afonso Rabelo Buzalaf. "Effect of iron on acid demineralisation of bovine enamel blocks by a soft drink." Archives of oral biology 2007; 52(11): 1109-1111.
- Pasdar, Nilgoon, Homayoon Alaghehmand, Fattane Mottaghi, and Maryam Tavassoli. "Experimental study of iron and multivitamin drops on enamel microhardness of primary tooth." Journal of International Society of Preventive & Community Dentistry 2015; 5(6): 518.
- Lökken, P., and J. M. Birkeland. "Dental discolorations and side effects with iron and placebo tablets." European Journal of Oral Sciences 1979; 87(4): 275-278.
- Addy, M., and J. Moran. "Extrinsic tooth discoloration by metals and chlorhexidine. II. Clinical staining produced by chlorhexidine, iron and tea." British dental journal 1985; 159(10): 331-334.
- Parnas, Limor, Mordechai Chevion, Eduard Berenshtein, Sarit Faibis, and Moti Moskovitz. "Are there metallic traces in black extrinsic dental stain?." Quintessence international 2013; 44(5).
- Shojaipour, R. "Adsorption Rate of Iron onto Primary Incisor Teeth Following the Application of Three Iron Drops." Journal of Kerman University of Medical sciences, 2015.
- Mehran, M. A. J. I. D., M. Mohammadi Basir, and S. E. D. I. G. H. E. H. Jafari. "Effect of two kinds of iron drops on the discoloration, atomic absorption and structural changs of primary teeth enamel." J Dent Med 2009; 21: 290-9.
- Shabzendehdar, Mahboubeh, Abbas Makarem, Hosein Orafai, Zahra Khashayarmanesh, and Saeed Ebrahimzadeh. "Comparsion of primary enamel discoloration caused by the use of three different iron drops (An in vitro study)." Journal of Mashhad Dental School 2006; 30: 247-254.
- Pani, Sharat Chandra, Fahad Murdhi Alenazi, Abdullah Muhammad Alotain, Hamad Daher Alanazi, and Abdullah Saeed Alasmari. "Extrinsic tooth staining potential of high dose and sustained release iron syrups on primary teeth." BMC oral health 2015; 15(1): 90.
- Stangel, I., E. Valdes, and J. Xu. "The absorption of iron by dentin: Its role in discoloration." Journal of Biomedical Materials Research: An Official Journal of The Society for Biomaterials and The Japanese Society for Biomaterials 1996; 31(2): 287-292.
- Hekmatfar, Somayeh, Hediyeh Piraneh, and Karim Jafari. "Evaluation of the relationship between pH and titrable acidity of five different of iron supplements with the absorption of iron ions in the anterior primary teeth (an in vitro study)." Dental research journal 2018; 15(5): 367.
- Garcia Martin, Jose Manuel, Manuel Gonzalez Garcia, Juan Seoane Leston, Santiago Llorente Pendas, Juan Jose Diaz Martin, and Maria Jose Garcia‐Pola. "Prevalence of black stain and associated risk factors in preschool S panish children." Pediatrics International 2013; 55(3): 355-359.
- Kato, Melissa Thiemi, Andrea Gutierrez Maria, Luís Guilherme Matiazi Vaz, Flávia de Moraes Italiani, Sílvia Helena de Carvalho Sales-Peres, and Marília Afonso Rabelo Buzalaf. "Effect of iron supplementation on the erosive potential of carbonated or decarbonated beverage." Journal of Applied Oral Science 2007; 15(1): 61-64.
- Reid, J. S., and J. A. Beeley. "Biochemical studies on the composition of gingival debris from children with black extrinsic tooth stain." Caries research 10, no. 5 (1976): 363-369.
- Surdacka, A. "Chemical composition of the saliva in children and adolescents with black tartar." Czasopismo stomatologiczne 1989; 42(10-12): 525-533.
APPENDIX
Figure 1. PubMed search history
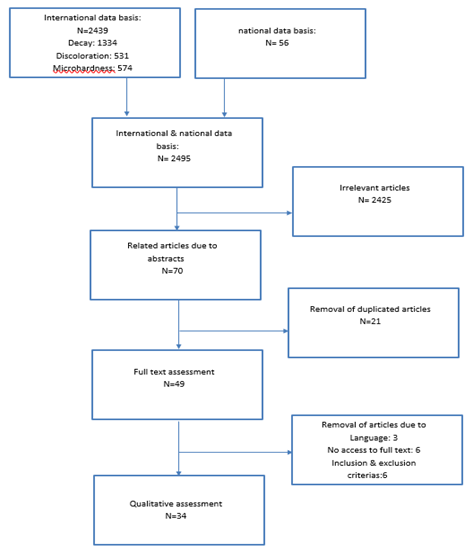
Figure 2. Search strategy
