Archive \ Volume.11 2020 Issue 1
Technological and Biopharmaceutical Aspects of Developing the Basics of Soft Medicinal Local Action
Victoria Tarasenko, Alexei Pidlisnyy, Alina Koval*, Andrew Solomennyy, Valentina Vaschuk, Lena Davtian, Natalya Goncharenko, Ivanna Sakhanda, Mariana Naumova
Ukrainian Military Medical Academy (Kyiv, Ukraine).

Abstract
Extensive clinical experience and experimental data irrefutably prove that local drug treatment of wounds should be built strictly in accordance with those processes that occur at different stages of the wound process, helping their natural course and not inhibiting it. The problem of treatment of purulent-inflammatory diseases, which is one of the oldest in surgery, remains acute at the present time. The basic principles of any method for treating purulent-necrotic processes are early removal of devitalized tissues, suppression of microflora activity in the lesion and acceleration of regeneration. The basis of soft drugs is of great importance since the effectiveness and speed of the onset of the therapeutic effect depends on this.
Keywords: soft medicinal products, local application, wounds

INTRODUCTION
The dosage form and the route of its introduction into the body has a significant impact on the pharmacotherapy of the disease since the action of the drug depends not only on the active pharmaceutical ingredient but also excipients included in the drug [1-4]. Studies in recent years on the development of the composition of multidirectional combined drugs in purulent wounds have been conducted mainly in the field of creating such dosage forms as ointments (creams), aerosols and drainage sorbents [5, 6]. It was established [7] that ointment is the most convenient for local wound healing dosage form, which has several advantages over other soft dosage forms. Thus, various hydrophilic and lipophilic drugs can be introduced into ointments, regulate their release and bioavailability of medicinal substances [8, 9]. A large number of bases allow the development of combination drugs, taking into account the features of the wound process in its various phases [3]. Ointments used for topical treatment of wounds in the first phase of the wound process must be hydrophilic-based, and have a long (up to 24 h) and pronounced osmotic activity [10, 11]. The most acceptable hydrophilic bases containing osmotically active preparations are gels [6, 12, 13].
The osmotic activity has various hydrophilic non-aqueous solvents (glycerol, propylene glycol, and others), polymers (polyethylene oxides, cellulose esters, acrylic acid derivatives, and others), having functional hydroxyl and carbonyl groups and capable of forming hydrogen bonds with water. Osmotic activity, including the mass of absorbed water and the time of its manifestation, depends on the nature of the base and substance, its molecular weight and concentration [6, 14].
Polyethylene oxides have been widely used in pharmaceutical technology as ointment bases. They have certain advantages over other excipients used in soft medicines [15]. Bases based on polyethylene oxides with different molecular weights are highly stable during storage, have weak bactericidal properties, and are well washed off with water [16]. A positive feature of polyethylene oxides is their low sensitivity to pH in a wide range of values, as well as to the introduction of electrolytes [7]. The combination of polyethylene oxides and some medicinal substances allows us to increase the dispersion of the latter, to achieve an increase in therapeutic effect at relatively low concentrations in the combined preparation.
The most common method in the treatment of purulent wounds in the first phase of the wound process is found to be gels consisting of polyethylene oxides-400 and polyethylene oxides-1500 in the ratio of 8:2. They are pharmacologically indifferent, easily applied to the wound surface and evenly spaced, improving the contact of the ointment with the tissues and the content of the wound, mix well with the wound exudate and retain their homogeneity [17]. Polyethylene oxides have a pronounced dehydrating effect not only on the tissues forming the bottom and walls of the wound cavity but also on the microbial cells present in the wound. Dehydration of a microbial cell leads to a significant decrease in its biological activity including resistance to the action of certain drugs. That is why in the presence of polyethylene oxides increases the antimicrobial action of antibiotics, antiseptics, and sulfanilamides, which are part of the ointment.However, it should be noted that polyethylene oxide ointment basics have a number of significant disadvantages, in particular, they dry the tissues and disrupt the barrier function of membranes, which in many cases is caused by the slow diffusion of polyethylene oxides-400 inside the cell [3]. The destruction of cell membranes, in turn, can lead to a sharp absorption of chemotherapeutic substances into the systemic circulation and the creation of "peak" concentrations there.
Today, a new generation of hydrophilic ointment bases have been developed for use in combination medicines intended for the treatment of wounds in the first phase of the wound process. They contain proxanol-268, polyethylene oxides-400, propylene glycol and water in various combinations [3, 18]. These bases have pronounced osmotic properties but do not have a damaging effect on the tissues due to the rapid penetration of the cells and provide an osmotic balance between the cytoplasm of tissue cells and ointment.The therapeutic tactics of the use of ointments in the first phase of the wound process is based on the fact that after elimination of significant purulent exudation, as well as after surgical treatment of volumetric wounds, the replacement of ointments with high osmotic activity for drugs with less pronounced, but long-lasting osmotic action, which is already should be minimized in the next group of drugs used in the second phase.
Without denying the possibility of using oily bases in the creation of ointments for the treatment of granulating wounds, it should be emphasized that the most optimal ones are the combined bases that provide a high effect of drug release, enhance their antibacterial and stimulating effect, as well as create a moderate level of dehydration in the wound. Two types of combined bases are particularly suitable for these requirements: first-viscosity plastic emulsions containing hydrophilic solvents and visco-plastic gels formed by hydrophilic surfactants and higher fatty alcohols comprising hydrophilic solvents and osmotically active polymers [7, 18]. The use of these bases opens up great opportunities for the creation of combination drugs since they can be hydrophilic and lipophilic drugs in the form of solutions, emulsions, and suspensions [3, 15].
To obtain oil/water emulsions with a grease-like consistency, a combination of certain first-order emulsifiers and higher fatty alcohols of an aliphatic series containing saturated alkyl chains with carbon numbers of at least 16-18 is used. In this case, the mechanism of stabilization of emulsions is associated with the creation in their volume of a structural-mechanical barrier, impeding flocculation and coalescence of oil phase droplets. This barrier is created by the formation of lipotropic liquid crystals from hydrophilic and lipophilic emulsifiers of molecules. Due to the anisometry, rigidity, and high partial concentration, they form a spatial grid in the volume of emulsions. Emulsions are coagulation structures with thixotropic properties, high structural viscosity, and yield strength.Oil/water type emulsions of oily consistency are obtained with different oil phases [3, 18]. Hydrophobic solvents are used as oil phases: petroleum jelly, sunflower oil, fish oil, benzyl benzoate, and ethyl-5 (condensation grade 15). Liquid hydrophobic substances, such as acol, sea buckthorn oil, and other carotenoid-containing drugs, are of interest in acting components.
In viscous-plastic emulsions of the first kind,quite high concentrations of hydrophilic solventscan be introduced: PEO-400, glycerol, 1,2-propylene glycol, and others. In addition, to certain concentrations, these solvents increase the structural viscosity and stability of the emulsions, reduce the moisture loss during storage, and lower the crystallization temperature of the dispersion medium.Thus, emulsions of the first kind are promising for use as the basis of drugs intended for topical use in the treatment of wounds in the second phase of the wound process. The rational choice of the composition and technology of the drug, which is applied to a particular stage of the wound process, may play a crucial role inthe maximum manifestation of therapeutic effect.
Local treatment of wounds remains one of the urgent problems of modern medicine. It is proved that irrespective of the genesis and localization of wounds, their healing proceeds according to the same biological mechanism during three phases of the wound process that consistently pass into each other. The effectiveness of local drug therapy in the use of different drugs depends on the differentiated use of drugs depending on the phase of the wound process.Practical medicine has a number of drugs for external use in the treatment of various wounds. But they do not fully meet the current requirements of clinicians, because in most cases they do not take into account the peculiarities of drug therapy of different phases of the wound process and have insufficient effectiveness. Improving the effectiveness of drugs for topical treatment of purulent wounds is possible through the development of new combination drugs intended for use in a particular phase of the wound process.
RESEARCH MATERIALS
When choosing a base, the physicochemical properties of the active pharmaceutical ingredients, stability, and ability to release from the ointment base were taken into account.Considering the medical and biological requirements for soft medicines for the treatment of wounds, we have used hydrophilic and hydrophilic-lipophilic bases with certain osmotic activity (depending on the stage of wounds) for the development of the dosage form. As hydrophilic excipients, polyethylene glycols of different molecular weight (polyethylene oxides 400, 1500, 6000), propylene glycol were used; high molecular weight compounds (polymers) - xanthan and guar gums, cellulose derivatives, polyvinylpyrrolidone, polyvinyl alcohol, polyacrylamide plasticizers - hydrophilic non-aqueous solvents (glycerol, propylene glycol, polyethylene glycol 400). For the construction of the hydrophilic-lipophilic base, we used surfactants (emulsifiers of the first and second kind), oil (mineral), and petrolatum (table 1).
RESEARCH METHODS
In order to select the optimal (in terms of medical and biological requirements) composition of the basics for soft drugs, we conducted studies on their thermo-colloidal stability, rheological properties, and osmotic activity.For determination of thermo-colloidal stability weused laboratory centrifuge MPW-210 of the company "Mechanika precyzyjna" (Poland) with a set of test tubes, a mercury thermometer with an interval of measured temperatures from 0 to 100 °C, split price -1 ° C, stopwatch, and water bath.Determination of stability was carried out according to the method described in the National Standard of Ukraine “Cosmetic creams. General Specifications: National Standard Specifications 4765: 2007[Valid from 2009-01-01]”[51]. A system that was not stratified by centrifugation for 5 minutes at 6000 rpm was considered stable. When heating a 10.0 gel in a well-sealed tube in a thermostat at 37 ± 1ºC for 24 hours, there should be no bundles (no coagulation, compaction, turbidity, and dilution). When freezing the gel sample in a test tube to -20ºC and subsequent gradual thawing at room temperature, there should be no bundles.
The osmotic properties of the experimental bases were studied gravimetrically using a dialysis method through a semipermeable membrane. A semipermeable membrane (cellophane film of brand B-8079 made by Cherkasy Chemical Fiber Plant) was attached to the lower opening of the inner cylinder of the dialysis chamber. The scheme of the dialyzer is presented in Fig. 1.
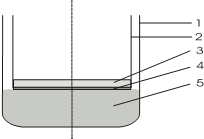
Figure 1. Dialysis scheme: 1- dialysis chamber; 2- internal cylinder; 3- sample; 4- semipermeable membrane; 5- purified water.
A sample of the soft drug under study (about 1.0 g) was applied uniformly to the surface of the semipermeable membrane, the area of which at a cylinder diameter of 50 mm was about 2000 mm2. The inner cylinder together with the sample was placed in a dialysis chamber into which a certain amount of purified water was poured in advance. The measurement of the mass of the inner cylinders was carried out every 60 minutes to constant weight on the analytical balance to the nearest 0.001 g, having previously wiped it from the outside. The tests were performed at a temperature of 37.0 ± 1.0 ºC using a TC-80M-2 thermostat. Periodically, the volume of water purified in the dialysis chamber was brought back to baseline [19]. The mass difference between the two weighings determined the amount of fluid absorbed.
Rheological studies were performed using the Rheotest-2 instrument (Germany).The shear stress was calculated using formula 1:
|
τr = zּ a |
(1) |
where:
τr – is the tangent shear stress, Paּ s;
z – is the constant of the instrument (depends on the type of cylinder);
a – is readings of the device.
After calculating the shear stress at the determined shear rates, the structural viscosity of the test bases was calculated using the formula 2:

η – is the structural viscosity, Pas;where: Dr – is the shear rate, s-1;
|
Table 1: Model basics for soft medicines |
||||||||||||||||||||
|
№ in order |
Name ingredients |
Model basics |
||||||||||||||||||
|
|
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
13 |
14 |
15 |
16 |
17 |
18 |
19 |
|
1. |
Vaseline |
20 |
60 |
|
|
40 |
30 |
20 |
|
|
|
10 |
|
|
|
|
|
|
|
|
|
2. |
Vaseline oil |
20 |
|
|
50 |
|
|
|
|
|
20 |
20 |
10 |
|
20 |
|
|
|
|
|
|
3. |
emulsifierТ-2 |
14 |
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4. |
Beeswax |
|
|
25 |
|
10 |
10 |
10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
5. |
Sunflower oil |
|
|
34 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6. |
Glycerol |
6 |
|
40 |
|
|
|
|
|
5 |
5 |
|
5 |
|
5 |
|
|
|
|
|
|
7. |
Twin 80 |
|
|
1 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8. |
Emulsion wax |
|
|
|
15 |
|
|
|
|
|
2 |
4 |
4 |
|
|
|
|
|
|
|
|
9. |
Aerosil |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1 |
2 |
|
10. |
PEO-400 |
|
|
|
|
|
|
|
70 |
|
5 |
10 |
10 |
|
|
28 |
5 |
|
|
|
|
11. |
PEO-1500 |
|
|
|
|
|
|
|
30 |
|
|
|
|
|
|
|
|
|
|
|
|
12. |
PEO-4000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
65 |
|
|
|
|
|
13. |
xanthan gum/guar 8: 2 |
|
|
|
|
|
|
|
|
1 |
|
|
|
1 |
1 |
|
|
|
|
|
|
14. |
Emulsifier 1 |
|
|
|
|
|
|
|
|
|
8 |
6 |
1 |
|
6 |
|
|
|
|
|
|
15. |
propylene glycol |
|
|
|
|
|
|
|
|
5 |
5 |
|
|
5 |
5 |
|
5 |
|
|
|
|
16. |
Carbopol940 Р |
|
|
|
|
|
|
|
|
|
|
|
1 |
|
|
|
1 |
|
|
|
|
17. |
Flogel 700 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0,47 |
0,45 |
|
|
|
18. |
Flocare ET58 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2 |
50 |
50 |
|
19. |
Triethanolamine |
|
|
|
|
|
|
|
|
|
|
|
0,65 |
|
|
|
0,65 |
|
|
|
|
20. |
The water is purified |
To 100 |
To 100 |
- |
To 100 |
To 100 |
To 100 |
To 100 |
- |
To 100 |
To 100 |
To 100 |
To 100 |
To 100 |
To 100 |
- |
To 100 |
To 100 |
To 100 |
To 100 |
DISCUSSION
The results of thermo-colloidal stability are shown in
table 2.
|
Table 2. Thermo-colloidal stability. |
|||
|
number in order |
Thermostability |
Colloidal stability |
|
|
|
Thermostat aging |
Freezing |
|
|
1, 2, 4, 9,13 |
+ |
+ |
- |
|
5, 6, 7 |
– |
+ |
+ |
|
14, 18, 19 |
+ |
– |
+ |
|
3, 8, 10, 11, 12, 15, 16, 17 |
+ |
+ |
+ |
Samples 3, 8, 15, 16, and 17 exhibited thermo-colloidal stability, so they were used for further studies. An important step in the development of soft drugs for external use is to study the rheological properties of carrier media (Tables 3-5, Figures 2-4).
|
Table 3. Rheological properties of samples No. 3 and 8. |
|||||||||
|
Dr, с-1 |
Shear stress, Pa |
Effective viscosity, Pa · s |
Dr, с-1 |
Shear stress, Pa |
Effective viscosity, Pa · s |
||||
|
|
Sample № |
|
Sample № |
||||||
|
|
3 |
8 |
3 |
8 |
|
3 |
8 |
3 |
8 |
|
3 |
56,8 |
34,1 |
18,9 |
11,4 |
1312 |
426,4 |
377,8 |
0,3 |
0,3 |
|
5,4 |
75,8 |
64,5 |
14,0 |
11,9 |
729 |
385,4 |
364,5 |
0,5 |
0,5 |
|
9 |
95,7 |
90,6 |
10,6 |
10,1 |
437,4 |
321,3 |
289,3 |
0,7 |
0,7 |
|
16,2 |
113,2 |
114,6 |
6,9 |
7,1 |
243 |
300,8 |
241,2 |
1,24 |
1,0 |
|
27 |
198,6 |
143,5 |
7,4 |
5,3 |
145,8 |
286,7 |
203,5 |
1,9 |
1,4 |
|
48,6 |
268,5 |
169,8 |
5,5 |
3,5 |
81 |
258,7 |
179,4 |
3,2 |
2,2 |
|
81 |
321,1 |
236,7 |
3,9 |
2,9 |
48,6 |
224,1 |
139,1 |
4,6 |
2,9 |
|
145,8 |
335,6 |
265,1 |
2,3 |
1,8 |
27 |
200,0 |
100,4 |
7,4 |
3,7 |
|
243 |
364,7 |
285,8 |
1,5 |
1,2 |
16,2 |
174,2 |
88,9 |
10,8 |
5,5 |
|
437,4 |
382,4 |
321,4 |
0,9 |
0,7 |
9 |
125,5 |
74,5 |
13,9 |
8,3 |
|
729 |
401,7 |
398,7 |
0,6 |
0,5 |
5,4 |
101,3 |
58,2 |
18,8 |
10,8 |
|
1312 |
426,4 |
383,2 |
0,2 |
0,3 |
3 |
84,5 |
31,2 |
28,2 |
10,4 |
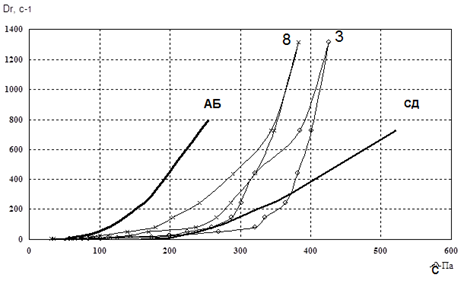
Figure 2. Reogram of samples No. 3 and No. 8
|
Table 4. Rheological properties of samples No. 10, 11, and 12. |
|||||||||||||
|
Dr, с-1 |
Shear stress, Pa |
Effective viscosity, Pa · s |
Dr, с-1 |
Shear stress, Pa |
Effective viscosity, Pa· s |
||||||||
|
|
Sample № |
|
Зразок № |
||||||||||
|
|
10 |
11 |
12 |
10 |
11 |
12 |
|
10 |
11 |
12 |
10 |
11 |
12 |
|
3 |
75 |
79 |
103 |
25 |
26 |
34 |
1312 |
321 |
341 |
341 |
0,2 |
0,3 |
0,3 |
|
5,4 |
98 |
85 |
128 |
18,1 |
15,7 |
23,7 |
729 |
259 |
244 |
314 |
0,4 |
0,3 |
0,4 |
|
9 |
113 |
114 |
138 |
12,5 |
12,7 |
15,3 |
437 |
239 |
203 |
265 |
0,5 |
0,5 |
0,6 |
|
16,2 |
136 |
137 |
156 |
8,4 |
8,5 |
9,6 |
243 |
213 |
173 |
214 |
0,9 |
0,7 |
0,9 |
|
27 |
159 |
168 |
184 |
5,9 |
6,2 |
6,8 |
145,8 |
185 |
132 |
201 |
1,3 |
0,9 |
1,4 |
|
48,6 |
183 |
191 |
191 |
3,8 |
3,9 |
3,9 |
81 |
158 |
96 |
183 |
1,9 |
1,9 |
2,3 |
|
81 |
201 |
226 |
217 |
2,5 |
2,8 |
2,7 |
48,6 |
102 |
84 |
165 |
2,1 |
1,7 |
3,4 |
|
145,8 |
224 |
248 |
235 |
1,5 |
1,7 |
1,6 |
27 |
95 |
72 |
147 |
3,5 |
2,7 |
3,0 |
|
243 |
248 |
263 |
254 |
1,0 |
1,1 |
1,0 |
16,2 |
78 |
65 |
121 |
4,8 |
4,0 |
7,5 |
|
437,4 |
271 |
297 |
287 |
0,6 |
0,7 |
0,7 |
9 |
62 |
52 |
102 |
6,9 |
5,8 |
11,3 |
|
729 |
299 |
321 |
332 |
0,4 |
0,4 |
0,5 |
5,4 |
42 |
48 |
83 |
7,8 |
8,9 |
15,4 |
|
1312 |
322 |
366 |
356 |
0,2 |
0,2 |
0,2 |
3 |
27 |
31 |
67 |
9 |
10,3 |
23 |
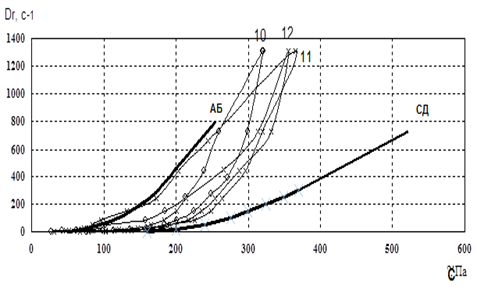
Figure 3. Reogram of samples No. 10-12.
|
Table 5. Rheological properties of samples No. 15, 16, and 17. |
|||||||||||||
|
Dr, с-1 |
Shear stress, Pa |
Effective viscosity, Pa · s |
Dr, с-1 |
Shear stress, Pa |
Effective viscosity, Pa· s |
||||||||
|
|
Sample № |
|
Зразок № |
||||||||||
|
|
15 |
16 |
17 |
15 |
16 |
17 |
|
15 |
16 |
17 |
15 |
16 |
17 |
|
3 |
134 |
98 |
87 |
44,7 |
32,7 |
29 |
1312 |
563 |
302 |
324 |
0,4 |
0,2 |
0,2 |
|
5,4 |
149 |
125 |
95 |
27,6 |
23,1 |
17,6 |
729 |
421 |
287 |
289 |
0,6 |
0,4 |
0,4 |
|
9 |
165 |
134 |
121 |
18,3 |
48,9 |
13,4 |
437 |
332 |
265 |
243 |
0,8 |
0,6 |
0,6 |
|
16,2 |
189 |
144 |
143 |
11,7 |
8,9 |
8,8 |
243 |
278 |
223 |
197 |
0,8 |
0,6 |
0,6 |
|
27 |
214 |
176 |
178 |
7,9 |
6,5 |
6,6 |
145,8 |
204 |
204 |
186 |
1,1 |
1,1 |
0,8 |
|
48,6 |
243 |
183 |
201 |
5 |
3,7 |
4,1 |
81 |
106 |
179 |
167 |
1,3 |
2,2 |
2,1 |
|
81 |
287 |
204 |
132 |
3,5 |
2,5 |
1,6 |
48,6 |
89 |
156 |
132 |
1,8 |
3,2 |
2,7 |
|
145,8 |
331 |
211 |
266 |
2,3 |
1,4 |
1,8 |
27 |
84 |
138 |
114 |
3,1 |
5,1 |
4,2 |
|
243 |
386 |
236 |
276 |
1,6 |
0,9 |
1,1 |
16,2 |
64 |
115 |
97 |
3,9 |
7,1 |
5,9 |
|
437,4 |
452 |
276 |
302 |
1,0 |
0,6 |
0,7 |
9 |
44 |
102 |
69 |
4,9 |
11,3 |
7,7 |
|
729 |
527 |
298 |
331 |
0,7 |
0,4 |
0,5 |
5,4 |
34 |
81 |
58 |
6,3 |
15 |
10,7 |
|
1312 |
579 |
321 |
378 |
0,4 |
0,2 |
0,3 |
3 |
23 |
72 |
45 |
7,8 |
24 |
15 |
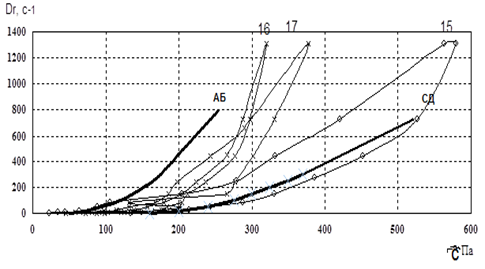
Figure 4. Reogram of samples No. 15-17.
Fig. 2-4 showed that the curve of samples 3 and 15 went beyond the rheological optimum. Therefore, these samples were excluded from our further studies.In the next stage of research, we studied the osmotic properties of samples 8, 10, 11, 12, 16, and 17 (Fig. 5).
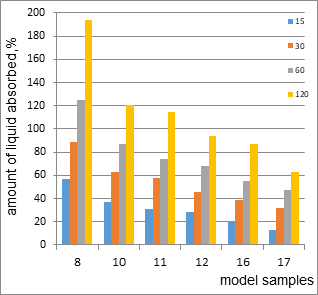
Figure 5. The osmotic activity of model specimens 8, 10-12, 16, and 17.
Comparative analysis of the data shows that sample 8 has the highest osmotic activity (194% after 120 min), due to the presence of high molecular weight and low molecular weight polyethylene oxides. Model samples 10-12 are characteristic of cream. Osmotic activity after 120 min for samples 10, 11, and 12are121%, 115%, and 94%.The osmotic activity of 121% in sample 10 is due to the presence of surfactants in the total amount of 12% and hydrophilic non-aqueous solvents (15%). Sample 11 differs from the composition of sample 10 by the presence of petroleum jelly, which leads to a slight decrease in the osmotic activity of the sample.The amount of surfactants in model 12 is 5%. The composition also included gel carbopol (1%). Therefore, the amount of liquid absorbed is less than 1.3 times that of sample 10. That is, the osmotic activity depends on the type and amount of excipients included in the model samples.Model samples 16 and 17 have a gel-like structure with an osmotic activity of 87% and 63%, respectively.
Based on the medical and biological requirements for the treatment of stage 2 and stage 3 wounds, we excluded model 8 (due to possible osmotic tissue shock). Among model samples 10-12, which are characteristic of the cream, we have selected samples 10 and 11, which correspond to the rheograms and consistency cream. However, given the amount of fluid absorbed and the stage of the wound(stage 2), we selected a model sample for further research.For the treatment of stage 3 wounds, it is optimal to use a gel. A comparative assessment of the osmotic activity of samples 16 and 17 showed the feasibility of selecting sample 16 with an osmotic activity of 87%.Thus, we have selected model samples 10 (cream) and 16 (gel) for further research.
CONCLUSIONS
- We chose the optimal (in terms of medical and biological requirements) composition of the basics for soft dosage forms.We conducted a study on their thermo-colloidal stability, rheological properties, and osmotic activity.These studies showed that samples 3, 8, 15, 16, and 17 exhibited thermo-colloidal stability, so they will be used for further studies.
- Our comparative analysis of the obtained data shows that sample 8has the highest osmotic activity (194% after 120 min), which is due to the presence of high molecular weight and low molecular weight PEO.
- We came to the conclusion that model specimens 10-12 are characteristic of cream, and model specimens 16 and 17 have a gel-like structure with an osmotic activity of 87%.
- We came to the conclusion that given the amount of fluid absorbed and the stage of the wound (stage 2), we will select a model sample (10) for further research.
- For the treatment of stage 3 wounds, the use of gel is optimal. A comparative assessment of the osmotic activity of samples 16 and 17 showed the feasibility of selecting sample 16 with an osmotic activity of 87%. Thus, we have selected model samples 10 (cream) and 16 (gel) for further research.
REFERENCES
- Abaev Yu. K. Spravochnik hirurga. Rany i ranevaya infekciya / Yu. K. Abaev. – Rostov n/D. : Feniks, 2006. – 427 s.
- Davtyan L. L. Vply`v farmacevty`chny`x faktoriv na anty`mikrobnu akty`vnist` kremu /L.L.Davtyan, T.F. Olifirova, S.V. Biryukova, O.B. Kolokolova // Farm.zhurnal. – 2010. – № 6. – S. 86 – 88.
- Percev I. M. Biologicheskaya farmaciya – sovremennaya teoriya optimalnogo proizvodstva i ispolzovaniya lekarstv / I. M. Percev, I.A. Zupanec // Klinichna farmaciya. – 1999. – T. 3, № 2. – S. 128 – 132.
- Zvyaginceva T. V. Lechebno-profilakticheskoe dejstvie mazi tiotriazolina pri mestnyh luchevyh povrezhdeniyah kozhi v eksperimente / T. V. Zvyaginceva, S. I. Mironchenko, E. V. Zhelnin //Eksperim. i klin. medicina. – 2009. – № 3. – S. 54–57.
- Drogovoz S. M., Drogovoz V. V. Farmakologiya na dopomogu likaryu, provizoru ta studentu. Pidruchny`k-dovidny`k. – X., 2004. – 476 s.
- Zubkov M. N. Prakticheskoe rukovodstvo po klinicheskoj mikrobiologii i antimikrobnoj terapii dlya vrachej stacionarnoj pomoshi / M. N. Zubkov. – M.: MGUP, 2002. – 412 s.
- Zmushko E. I., Belozerov E. S. Medikamentoznye oslozhneniya.— SPb: Piter, 2001. – 448 s.
- Imasheva A. K. Osobennosti regeneratornyh processov kozhi pri termicheskih ozhogah /A. K. Imasheva, M. V. Lazko // Fundamentalnye issledovaniya. – 2009. – № 5. – S. 22–24.
- Paramonov B.A. Maz «Soderm» dlya lecheniya ozhogovyh ran / Paramo.nov B.A., Turkovskij I.I., Shlyahov A.M., Churilova I.V., Drozdova Yu.I., Leonova N.V., Malahov S.F., Karnovich A.G. // Sovremennye metody mestnogo medikamentoznogo lecheniya obozhzhennyh. Nizhegorodskij medicinskij zhurnal (prilozheniya «Kombustiologiya»). – 2004. – S. 142–143.
- Astahov A.V. Neblagopriyatnye pobochnye reakcii i kontrol bezopasnosti lekarstv / A.V. Astahov, V.K. Lepahin // Rukovodstvo po farmakonadzoru.— M.: Kognito-Centr, 2004. – 200 s.
- DerzhavnafarmakopeyaUkrayiny` / Derzhavnepidpry`yemstvo „Naukovo-ekspertny`jfarmakopejny`jcentr”. – 1-evy`d. – X. : RIREG, 2004. – Dop. 1. – 520s.
- Chadaev A. P. Sovremennye metodiki mestnogo medikamentoznogo lecheniya inficirovannyh ran / A. P. Chadaev, A.D. Klimiashvili // Hirurgiya. – 2003. – № 1. – S. 43 – 56.
- Farmacevticheskie i mediko-biologicheskie aspekty lekarstv. V 2-h tomah / I. M. Percev, I. A. Zupanec, L. D. Shevchenko – X: Izd-vo UkrFA, 1999. – T. 1, 2. – 464 s.; 448 s.
- Garijon Zhan-Lui Kvantovaya medicina - medicina zavtrashnego dnya / Garijon Zhan-Lui, A.Ya. Grabovshiner // II Mezhdunarodnyj kongress "Slabye i sverhslabye polya i izlucheniya v biologii i medicine": Trudy . – SPb: Respublikanskoe izdatelstvo "Licej", 2000. – S. 78 – 83.
- Racionalnaya antimikrobnaya farmakoterapiya: Rukovodstvo dlya praktikuyushih vrachej /Pod red. V. P. Yakovleva, S. V. Yakovleva. – M., 2003. – 261 s.
- Minchenko A. N. Rany, lechenie i profilaktika lechenij / Pod red. N. V. Ruhlada. – SPb.:, 2002. – 39 s.
- Ehrenkranz N. J. Antimicrobial Prophylaxis in Surgery: Mechanisms, Misconceptions, and Mischief / N. J. Ehrenkranz // Infection Control and Hospital Epidemiologi. –1993. – V. 14, № 2. – P. 99–106.
- Kremy` kosmety`chni. Zagal`ni texnichni umovy`: DSTU 4765:2007. – [Chy`nny`j vid 2009–01–01]. – K. : Derzhspozhy`vstandart Ukrayiny`, 2008. – 7 s. – (Nacional`ny`j standart Ukrayiny
- Davtyan L. L. Vy`vchennya osmoty`chny`x vlasty`vostej model`ny`x osnov zalezhno vid nosiya / L. L. Davtyan // Farmacz. zhurn. – 2003. – №3. – S. 74 – 77.
