Archive \ Volume.11 2020 Issue 2
Analysis of the Ukrainian Market of Antibacterials for Systemic Use for the Treatment of Military Personnel with Craniocerebral Injuries
Oleksandr Shmatenko, Olga Plieshkova, Oksana Bielozorova, Viktoria Shmatenko, Anna Drozdova, Vladyslav Strashnyi, Mykola Ponomarenko , Alina Koval*
Ukrainian Military Medical Academy, Kyiv, Ukraine.
Abstract
The article presents the results of comparative marketing analysis of the antibacterials for systemic use, that are used for the pharmacotherapy of traumatic craniocerebral injuries of military personnel, by the number of international nonproprietary names, brand names, types of dosage forms and price proposals.
Keywords: antibacterial agents, military, craniocerebral trauma
INTRODUCTION
Health is considered as a necessary field of science since many years ago [1-3]. Traumatic craniocerebral injury is one of the leading causes of death and disability. Yearly for every 10 thousand population, there are 200 cases of the trauma, [4-7], and in Ukraine, more than 11 thousand people die per year, i.e. mortality from head injuries is 0.024% [4, 8-10]. Also, this is an urgent problem for the medical service of the Armed Forces of Ukraine: according to the scientific literature, craniocerebral injuries in large-scale wars and local armed conflicts of the twentieth century ranged from 7.9% to 17.9% of all injuries [4, 8, 9, 11, 12], and during the Joint Forces operation and the Anti-Terrorist operation in eastern Ukraine, head injuries accounted for 31.9% [8, 9, 11, 12]. Also, the treatment of military personnel with trauma requires the use of a significant number of medicines, among which a special role is played by antibacterials for systemic use. They are used to prevent infectious complications. The effective usage of antibiotics in neurosurgery has specific requirements for medicine’s ability to penetrate the blood-brain barrier with conventional parenteral administration and not cause side complications when applied typically to a brain wound and injected into the cerebrospinal fluid [4, 13, 14]. Antibiotics of the tetracycline group, penicillins, cephalosporins, macrolides, lincosamides, aminoglycosides, etc. meet these requirements.
Replenishment of the pharmaceutical market of Ukraine with new medicines, both domestic and foreign, makes changes to the market structure. Given this, the analysis of the pharmaceutical market of modern antibacterial agents for systemic use remains relevant today. Therefore, the purpose of our work is to conduct a comparative analysis of the range of modern antibacterial agents for systemic use, presented in the pharmaceutical market of Ukraine.
MATERIALS AND METHODS OF RESEARCH:
During the research process, we used materials from the data of 179 prescription sheets and also cards of inpatients with trauma who were treated at the National Military Medical Clinical Center "Main Military Clinical Hospital". Research methods: marketing analysis, systematic analysis, bibliographic overview.
RESULTS OF THE RESEARCH AND DISCUSSION OF THE RESULTS:
According to the ATC classification, antibacterials for systemic use belong to group J – anti-infectives for systemic use, and include such groups as J01A - tetracyclines, J01B - amphenicols, J01C - beta-lactam antibiotics, penicillins, J01D - other beta-lactam antibiotics,
J01E - sulfonamides and trimethoprim, J01F - macrolides, lincosamides, and streptogramins, J01G - aminoglycosides, J01M - quinolone antibacterial agents,
J01R - combined antibacterial agents and J01X - other antibacterial agents. During the analysis of primary medical records and scientific literature was found that the following agents are used to treat trauma in military personnel: pefloxacin (J01MA03), tobramycin (J01GB01), amikacin (J01GB06), amoxicillin (J01CA04), benzylpenicillin (J01CE01), doxycycline (J01AA02), gentamicin (J01GB03), erythromycin (J01FA01), kanamycin (J01GB04), lincomycin (J01FF02), netilmicin (J01GB07), nitroxoline (J01XX07), norfloxacin (J01MA06), ofloxacin (J01MA01), cefotaxime (J01DD01), ceftazidime (J01DD02), ceftriaxone (J01DD04), ciprofloxacin (J01MA02).
The analysis of registered in Ukraine antibacterial agents for systemic use for the medical supply of servicemen with trauma was conducted using the information database of registered medicines, presented on the official website of the State Expert Center of the Ministry of Health of Ukraine, and showed that as of 01.12.2013 in Ukraine have been registered 330 antibiotics, which are used to prevent infectious complications in military personnel with trauma. During the comparative analysis as of 01.12.2017 and 01.06.2020, it was found that the number of medicines decreased and amounted to 301 and 273, respectively (Fig. 1).
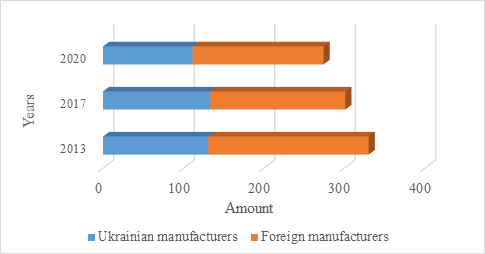
Fig. 1. Distribution of medicines by manufacturer
Analyzing the origin countries, it was found that foreign companies produced 200 medicines in 2013, but over the next four years, the number of foreign medicines decreased to 168 items, and in 2020 - to 162. The leader in each 2013, 2017, and 2020 was India, which supplied 72, 68, and 57 items to the domestic pharmaceutical market, respectively (Fig. 2). Also, the top five include Slovenia, Austria, Italy, and Germany, whose total import in 2020 counts as 57.
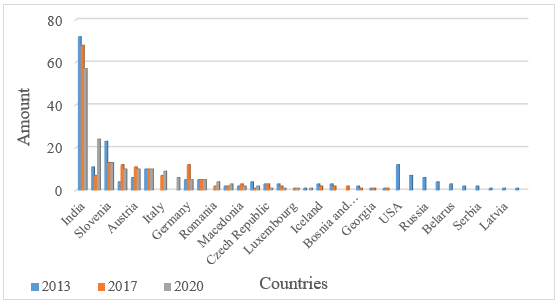
Fig. 2. Distribution of medicines by origin countries
Generally, in June 2020, the number of medicines produced by foreign manufacturers decreased by 18% due to reduced imports from the United States, Canada, Russia, and others.
As of 2020, Ukrainian manufacturers supply 111 medicines. The top three is headed by PJSC "Pharmaceutical Company Darnytsia", which produces 19 items and has moved OJSC "Kyivmedpreparat" out of the first place. The second place is taken by scientific production center PJSC "Borshchahivsky HFZ" (16 items), and the third place is occupied by OJSC "Kyivmedpreparat" (15 items) (Fig. 3).
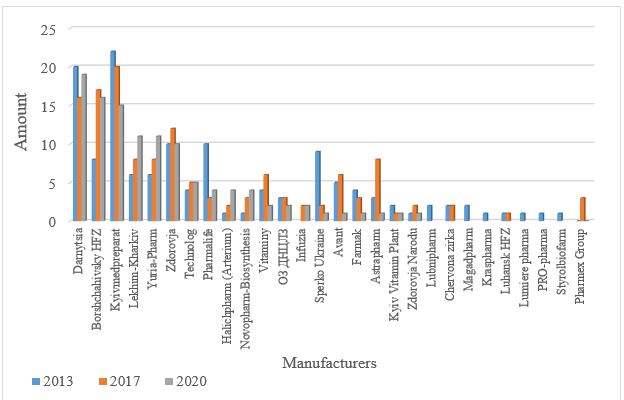
Fig. 3. Distribution of antibacterial agents for systemic use by manufacturers
During the analysis of fig. 3, it is found that 7 manufacturers have expanded the range of produced medicines from 1.25 up to 4 times: PJSC scientific production center "Borshchahiv HFZ", LLC "Lekhim-Kharkiv", LLC "Yuria-Pharm", LLC "Technology", JSC "Technology Halychpharm", "Novopharm-Biosynthesis" LLC and "Infuzia" LLC. All other domestic pharmaceutical companies have reduced production due to the crisis caused by the Joint Forces operation and the anti-terrorist operation in the east of the country, and some manufacturers, such as OJSC "Lubnipharm", OJSC "Chervona Zirka", LLC "Luhansk HFZ", "Styrolbiofarm" LLC, "Pharmex Group" LLC, stopped production of antibacterial agents for systemic use at all.
The analysis of antibacterial agents for systemic use under international nonproprietary names (INN), presented in Fig. 4, showed that the widest range in each 2013, 2017 and 2020 was characterized by ceftriaxone
(J01DD04) - their number over the past seven years increased by 34% and currently stands at 87. In 2013, Bangladesh, Belarus, Bulgaria, Great Britain, Georgia, India, Iran, Cyprus, Russia, the USA, and Switzerland were engaged in its production. In 2017, Luxembourg, Germany, and Turkey joined the production of ceftriaxone medicines, and in 2020 - Italy, China, and Romania. Among domestic pharmaceutical companies, ceftriaxone is currently produced by OJSC "Kyivmedpreparat", PJSC "Pharmaceutical Company Darnytsia", CJSC "Lekhim-Kharkiv", scientific production center PJSC "Borshchahivsky HFZ", LLC "Zdorovya", and LLC "Yuria-Pharm".
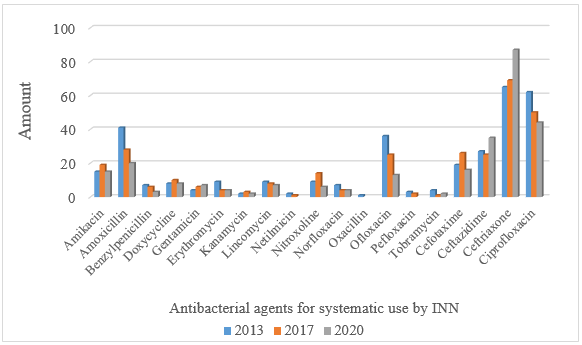
Fig. 4. Distribution of antibacterial agents for systemic use by INN
The second place, despite the reduction of the range by 30%, is occupied by ciprofloxacin (J01MA02) - 44 items. The third place is taken by ceftazidime (J01DD02) - 35 items. Also in the top five are amoxicillin (J01CA04) and cefotaxime (J01DD01), with 20 and 16 items, respectively.
After segmentation process of the range of antibacterial agents for systemic use by type of dosage form, it was found that in 2013 the largest number of antibiotics was produced in the form of injection, such as powders for solutions for injection - 128, solutions for injections - 32, solutions for infusion - 15 and concentrates for solution for injection - 2; tablets: coated tablets, dispersed, soluble, prolonged action - 103; capsules - 23; other, namely: eye/ear drops - 14 items, powders for suspension - 10, ointments - 3. In 2020, the number of injectables increased to 189, which is 69% of the total range of medicines. This is due to the increase in the production of cephalosporins, which are produced in the form of powders for solutions for injection (Fig. 5).
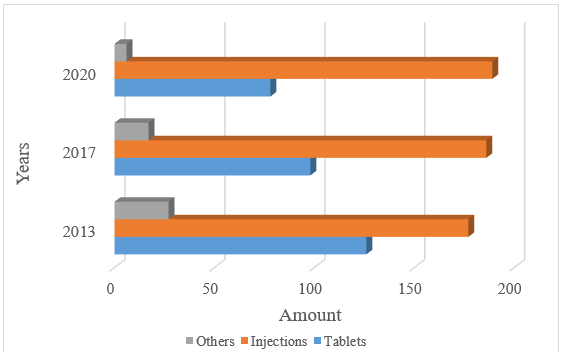
Fig. 5. Distribution of antibacterial agents for systemic use by type of dosage form
Analysis of the cost per a single dose of antibacterials for systemic use gave a possibility to found that, in general, the price of foreign-made medicines is much higher compared to the cost of domestic one (Table 1).
|
Table 1: The average cost per a single dose of antibacterial agents for systemic use |
|||
|
ATC-code |
INN |
Price, UAH |
|
|
|
|
Ukrainian manufacturers |
Foreign manufacturers |
|
J01GB06 |
Amikacin |
40,21 |
48,25 |
|
J01CА04 |
Amoxicillin |
11,44 |
25,71 |
|
J01CE01 |
Benzylpenicillin |
7,96 |
20,86 |
|
J01GB03 |
Gentamicin |
2,23 |
3,05 |
|
J01AA02 |
Doxycycline |
0,91 |
11,18 |
|
J01FA01 |
Erythromycin |
0,75 |
|
|
J01GB04 |
Kanamycin |
14,70 |
25,18 |
|
J01FF02 |
Lincomycin |
3,01 |
78,80 |
|
J01GB07 |
Netilmicin |
600,00 |
|
|
J01ХХ07 |
Nitroxoline |
0,27 |
3,14 |
|
J01МА06 |
Norfloxacin |
13,97 |
|
|
J01МА01 |
Ofloxacin |
17,60 |
47,13 |
|
J01МА03 |
Pefloxacin |
15,80 |
|
|
J01GB01 |
Tobramycin |
37,67 |
|
|
J01DD01 |
Cefotaxime |
12,70 |
67,95 |
|
J01DD02 |
Ceftazidime |
26,10 |
91,69 |
|
J01DD04 |
Ceftriaxone |
16,99 |
63,85 |
|
J01МА02 |
Ciprofloxacin |
20,88 |
29,81 |
The difference between the cost of medicines ranged from 1.2 times more for amikacin (J01GB06) and up to 26,2 times more - for lincomycin (J01FF02).
CONCLUSIONS:
The marketing analysis was performed for the antibacterials for systemic use, which are used for the medical supply of military personnel with craniocerebral injuries, by the number of INN, production, type of dosage form and cost.
- During the analysis by origin country, it was determined that India was the leader in the production of antibacterial agents for systemic use in 2013 and the next seven years (72, 68 and 57 items, respectively). Also, the top five include Slovenia, Austria, Italy, and Germany.
- During production analysis, it was found that the market share of domestic antibacterial agents for systemic use is 41%. Among Ukrainian manufacturers, the leading positions are taken by PJSC "Pharmaceutical Company Darnytsia" (19 items), PJSC scientific production center "Borshchahivsky HFZ" (16 items) and OJSC "Kyivmedpreparat" (15 items).
- INN analysis showed that the first place among the antibacterial agents for systemic use by the number of trade names is ceftriaxone (J01DD04) (87 items), on the second place is ciprofloxacin (J01MA02) (44 items). Third place is taken by ceftazidime (J01DD02) - 35 items. Also in the top five are amoxicillin (J01CA04) and cefotaxime (J01DD01) - 20 and 16 items, respectively.
- The distribution of antibacterials for systemic use by type of dosage form has some changes: the number of injectables has increased significantly to 189 items (69% of the total range).
- Analyzing the cost of a single dose of antibacterial agents for systemic use, it was found, that generally, the price of medicine of foreign production is much higher than the cost of domestic up to 26,2 times more.
REFERENCES
- Hanawi SA, Saat NZ, Zulkafly M, Hazlenah H, Taibukahn NH, Yoganathan D, Abdul Rahim NN, Mohd Bashid NA, Abdul Aziz FA, Low FJ. Impact of a Healthy Lifestyle on the Psychological Well-being of University Students. International Journal of Pharmaceutical Research & Allied Sciences. 2020 Apr 1;9(2).
- Alzaid A, Alosaimi M, Alkahtani KF, Alshehri BA, Asiri AE, Asiri AM, Althibait SA, Aldrees WS, Althwaiqub AK, Almakhayitah OA, Almarzooq MJ. Saudi Parents' Knowledge, Attitudes, and Practices Regarding Antibiotic use for Upper Respiratory Tract Infections in Children. International Journal of Pharmaceutical Research & Allied Sciences. 2020 Jan 1;9(1):115-20
- Ghiasi N, Hidarnia A, Niknami S, Motlagh ME. The impact of marriage enrichment program via distance learning on mental health and its dimensions among Iranian newly-wed couples: a six-month follow-up. J. Adv. Pharm. Educ. Res | Oct-Dec. 2018;8(S2):89-99.
- Konovalov AN, Likhterman LB, Potapov AA. Clinical guidelines for traumatic brain injury, 1 & 3. Moscow,“Antidor. 1998.
- Polishchuk M.Є. Standardization of the disease. What is the best idea for neuroprotectors in cerebrovascular pathology and traumatic brain injury? I think so. International neurological journal. Kharkiv, 2014; 6 (68). S. 49-53.
- Cherednichenko T.V., Bayun Yu.V., Bukriy A.O. Management of patients with closed craniocerebral injury in the acute period (clinical case). Skhidno-European neurological journal. 2019. No.1. S. 30-35.
- Maltsev, L.A., Grishin, V.I., Bazilenko, D.V., Pshenko, S.O. Traumatic brain injury: intensive care, monitoring, threshold values of targets. Emergency medicine. Kiev, 2018. No. 1: 72-75.
- Danchin, A.G. Treatment tactics for gunshot wounds to the skull and brain in the current military conflict, Ukrainian journal of minimally invasive and endoscopic surgery. Kiev, 2015; 19(1): 15–23.
- Sirko A.G. Vognepalny wounds to the skull and brain before an hour of great confrontation at the gathering of Ukraine. Occasional 2. Hirurgical treatment. Ukrainian neurosurgical journal. Kiev, 2015. No. 2. S. 46-53.
- Bornstein N. Poon W.S. Clinical efficacy of neurotrophic treatment in stroke and traumatic brain injury. Drugs Today. 2012. V. 48, suppl. A. P. 43-61.
- Ya.L. Zarutsky, V.M. Zaporozhana. Military-polova surgery: handler, Odessa: ONMedU, 2016.416 p.
- Polishchuk M.Y., Honcharuk O.M. The traumatic brain injury is closed. An awful glance at the problem. International neurological journal. Kharkiv, 2015. 6 (76). S. 57-65.
- Yovenko І.A. Bacterial control in severe exogenous trauma. Medicine of non-emergency staniv. Kiev, 2015. No. 2. S. 171-175.
- Dellinger R.P. Levy M.M., Rhodes A. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. CritCareMed. 2013. No. 41. R. 580-637
