Archive \ Volume.11 2020 Issue 3
Biodiesel Production from Waste Cooking Oil Using Catalyst Calcium Oxide Derived of Limestone Lintau Buo
Vivi Sisca, Syukri, Zilfa, Novesar Jamarun*
Department of Chemistry Andalas University Kampus Unand Limau Manis, Padang Indonesia.

Abstract
Biodiesel is an alternative to fossil fuels. It is produced by transesterification of triglycerides with short-chain alcohols in the presence of a catalyst. Sources of triglycerides include vegetable oils and animal fats. Among alkali metal oxide catalysts, calcium oxide (CaO) is widely used in the transesterification reaction. CaO is strongly alkaline and is cheap and environmentally friendly. It has low solubility in methanol. CaO can be sourced from natural limestone deposits. In this study, a solid CaO catalyst was obtained from Lintau Buo limestone and calcined at 900°C for 5 h. The results showed that this catalyst had a significant effect on biodiesel production.
Keywords: CaO, Heterogeneous catalyst, Transesterification, Waste Cooking Oil, Biodiesel
INTRODUCTION
Renewable and environmentally friendly fuels are being developed as a consequence of the scarcity of fossil fuels, global warming, and environmental problems [1-3]. Biodiesel, fatty acid methyl ester, or FAME, is a monoalkyl ester of fatty acids from vegetable oils or animal fats are an alternative solution [4, 5]. Its biodegradability, renewability, and physical similarity to diesel petroleum but with less toxic emissions make it a feasible sustainable energy source [6, 7].
FAME is produced by the transesterification of vegetable oils or animal fats with short-chain alcohol in the attendance of a catalyst [8]. The use of land to produce vegetable oils for FAME reduces that available for food production which can cause social problems. To minimize the effect on food production, waste cooking oil can be used as an alternative raw material making the process efficient and effective in minimizing environmental impact [9].
Homogeneous catalysts like NaOH, KOH, and H2SO4 are effective for transesterification [10, 11], producing a high yield in short reaction time. Nevertheless, homogeneous catalysts can result in problems such as saponification during the reaction, production of large amounts of wastewater, corrosion due to emulsification, difficulty in separation, and side reactions such as decomposition and polymerization [12]. The employment of heterogeneous catalysts can reduce some of these problems.
Heterogeneous catalysts are easily separated. They are recyclable, environmentally friendly, and do not produce large amounts of wastewater [13]. Heterogeneous transesterification catalysts that have been studied include alkaline earth metal oxides, alkali metal compounds supported on alumina, zeolites, solid acids, and enzymes Previous studies have reported that alkaline earth oxides which have high alkalinity can be used to produce FAME [14]. The most frequently used heterogeneous base catalyst is calcium oxide (CaO) [15]. CaO is low-cost, long-lived, moisture resistant, and has low solubility in a liquid mixture. Also, it produces a high yield of FAME [14].
CaO is widely used as a heterogeneous catalyst because there are many natural sources of calcium available. Natural calcium carbonate from eggshells, shells, limestone, dolomite, cuttlebone, or other sourced can be converted to CaO via calcination [16].
The presence of high amounts of calcium in lime makes it a potential source of CO for a heterogeneous catalyst. However, little research has been conducted on the potential of heterogeneous base catalysts derived from natural limestone sources. Hence, low-cost heterogeneous base catalysts synthesized from local limestone is the focus of this study.
The source of limestone used is the Lintau Bou area of West Sumatra. This limestone contains Ca (97%) along with Mg (0.5%), Al (0.4%), (0.3%), Fe (0.1%), Ag (1.1%). Morphological and elemental properties of the calcinated limestone were characterized by SEM and XRF. Structural properties were identified through XRD analysis. Catalytic activity was evaluated through a transesterification reaction with waste cooking oil (WCO).
MATERIAL AND METHODS
Material
Limestone was collected from Lintau Buo Regency; West Sumatra. Waste cooking oil (used three times for frying) was collected from fried food vendors in Jati Padang, West Sumatra, and then analyzed for chemical composition using GCMS. Methanol, ethanol, and n-hexane were purchased from Merck Limited, Padang, Indonesia.
Catalyst Preparation
Limestone was rinsed using distilled water to remove surface impurities and dried at 105°C. It was then crushed using a mortar and calcined at 900°C for 5 h in a furnace to produce CaO which was mashed using a mortar and placed in an airtight container to avoid contact with CO2 and H2O.
Characterization of waste cooking oil and catalyst
WCO was filtered with Whatman filter paper no. 42 to remove sludge. After that, the WCO was dried at 100°C to constant weight. The fatty acid composition of WCO was analyzed by GCMS. The crystal structure of the catalyst before and after transesterification was determined using X-ray diffraction (XRD) by PanAnalytical Expert Pro which uses Cu Kα radiation. Elemental analysis was performed by X-ray fluorescence (XRF) analysis using the PANalytical Epsilon 3 analysis model. Morphological characteristics were analyzed using scanning electron microscopy (SEM) and X-ray spectroscopy (EDX) dispersive energy (JX-J), JSM-6290LV.
Transesterification
The transesterification was conducted in a 500 ml three-neck flask equipped with a reflux condenser and a magnetic stirrer. First, methanol and catalyst were added to the flask and heated to the reaction temperature. Then WCO was added and heated at 50°C while stirring at 850 rpm. Amount of catalyst was varied (1, 3, 5, 7 wt%) as was the molar ratio of methanol: oil (3:1, 6:1, 9:1, 12:1), reaction temperature (55, 60, 65, 70°C) and reaction time (2, 4, 6, 8 h). After the reaction was completed, the solid catalyst was filtered out using a funnel complete with filter paper. The filtrate was transferred to a separating funnel and left overnight. The top layer was collected and washed with hot distilled water until clear. The FAME was dried at 110°C to remove the remaining washing water, and the FAME content was analyzed by gas chromatography and yield calculated using the following equation:
Methyl ester  100% [17]
100% [17]
RESULTS AND DISCUSSION
Determination of the content of free fatty acids
Based on (figure1) linoleic fatty acid content was 49.56% (Table 1). Mahreni (2010), found a simlar sample contained 55% linoleic acid [18, 19].
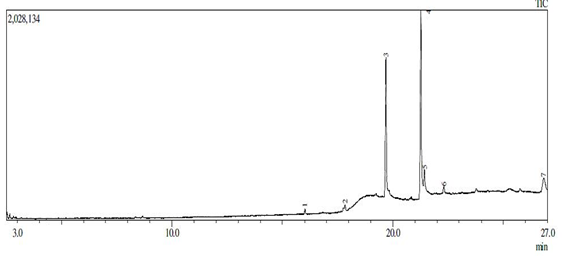
Figure 1. Chromatogram of waste cooking oil from Gas Chromatography
|
Table 1. Peak Report |
||||
|
Peak# |
R.Time |
Area% |
Height |
|
|
1 |
16.017 |
0.86 |
46322 |
Palmitate |
|
2 |
17.834 |
1.09 |
53076 |
Oleate |
|
3 |
19.688 |
31.73 |
1339759 |
Palmitin |
|
4 |
21.270 |
49.56 |
1809337 |
Linoleic |
|
5 |
21.430 |
7.49 |
234934 |
Stearin |
|
6 |
22.310 |
1.54 |
61366 |
Hexadecanoic |
|
7 |
26.836 |
7.72 |
124911 |
Borane |
X-ray diffraction (XRD) analysis
The XRD pattern of the limestone (Figure 2a) shows the presence of CaCO3 peaks at 2θ: 23°, 29°, 35°, 39°, 43°, 47°, and 48° (ICDD Reference No: 01-078-4614). It can be seen that the main component of limestone is calcium carbonate. These results are consistent with XRF analysis, which also indicates that calcium (Ca) is the main element. Peaks corresponding to CaO and Ca (OH)2. CaO at 2θ: 32°, 37°, 53°, 64°, 67°, 91° (ICDD Reference Number: 01-077-2010) and Ca (OH)2 at 2θ: 17°, 28°, 34°, 46°, 50°, 54°, 62° (ICDD Reference Number: 01-084-1265) can also be observed (Figure 2b). This indicates that after calcination this limestone should produce CaO with high crystallinity. Above 700°C, CaCO3 turns into CaO and 900°C is the optimal temperature for calcination [20]. The appearance of the Calcium diglyceroxide at 2θ: 20° (ICDD Reference No: 00-021-1544) peak after CaO has been used in transesterification (figure 2c) corresponds to its formation in the process of combining calcium oxide with glycerol [21]. Calcium diglyceroxide is an active species that can function as a catalyst in the transesterification reaction [22].
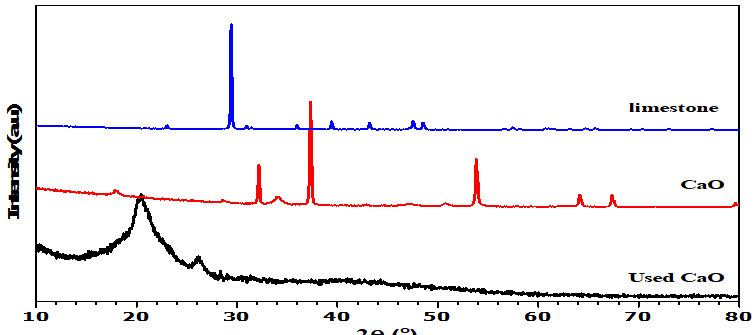
Figure 2. X-ray diffraction analysis (a) Limestone (b) CaO (c) Used CaO
X-ray fluorescence (XRF) analysis
The composition of the limestone and CaO were analyzed by XRF (Table 2). Ca content in limestone is 97%, while Ca content is 98% in CaO after calcination. Ca content decreased to 93% after the first transesterification reaction cycle. A small amount of the CaO catalyst reacted with methanol to form Ca (C3H7O3)2 and Ca (OH)2, but most was able to be recovered as CaO(20).
|
Table 2. X-ray fluorescence analysis (a) limestone (b) CaO (c) Used CaO |
|||||
|
Limestone |
CaO |
Used CaO |
|||
|
Compound |
Conc |
Compound |
Conc |
Compound |
Conc |
|
Mg |
0,5% |
Mg |
0,3% |
Mg |
1,7% |
|
Al |
0,4% |
Al |
0,4% |
Al |
0,7% |
|
Si |
0,3% |
Si |
0,4% |
Si |
0,8% |
|
Ca |
97% |
Ca |
98% |
P |
1,7% |
|
Fe |
0,1% |
Fe |
0,1% |
Ca |
93% |
|
Ag |
1,1% |
Ag |
0,9% |
Fe |
0,4% |
|
|
|
|
|
Ag |
1,4% |
SEM analysis
(Figure 3) shows an SEM of the CaO catalyst before and after the transesterification reaction showing morphological changes after the reaction. Fresh catalyst has a uniform and irregular distribution of particles, whereas used catalyst show particles agglomerated with other reactant molecules. Morphological changes occur due to the blockage of active sites with by-products such as diglycerides, monoglycerides, glycerol, and FAME and contamination by moisture and CO2 in the air during the catalyst filtration process [23]. These physical changes also contribute to a decrease in catalytic performance. The morphological changes to a catalyst after the first reaction is known to result in a new active surface which has a significant impact on catalytic activity [24].
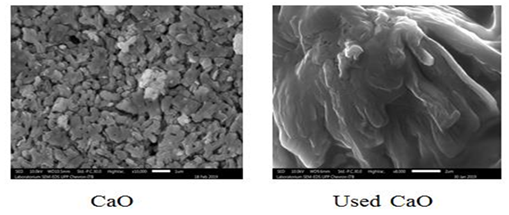
Figure 3. SEM Analysis (a) CaO (b) Used CaO
Amount of catalyst
The effect of the amount catalyst on transesterification was significant (Figure 4). When other conditions were constant (molar ratio alcohol/oil 6:1, 65°C, 4 h) a small amount of catalyst produced a low yield of FAME. Optimal results were obtained at 5wt% CaO which produced a 86% yield. Higher amounts of CaO than this reduced the yield. A critical mass of catalyst is needed to drive a reaction [25, 26]. On the other hand, too much catalyst makes the reactant mixture too thick, which causes problems with mixing and separation [27].
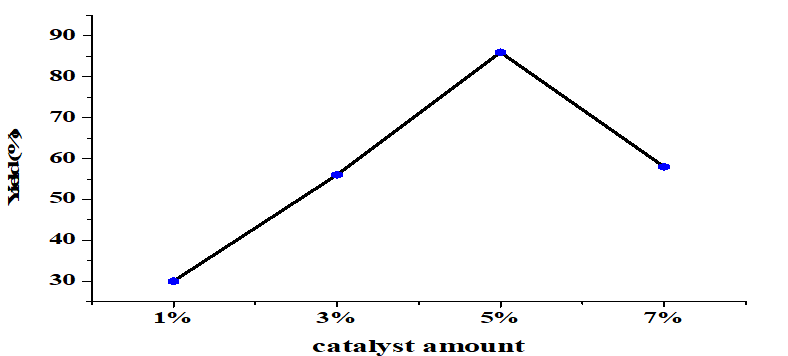
Figure 4. Yield for different amounts of catalyst
Methanol to oil ratio
The molar ratio of alcohol/oil in the transesterification stage under constant conditions (5wt% catalyst, 65°C, 4h) (Figure 5), had a significant effect on yield which increased with increasing methanol/oil ratios up to 6: 1 (yield of 86%). Exceeding this optimum value reduced yield. Stoichiometry of the transesterification reaction indicates that one mole of oil (or triglyceride) needs three moles of alcohol to produce three moles of FAME and one mole of glycerol. Because transesterification is reversible, excess methanol is required to move the equilibrium towards the formation of methyl esters [28]. Large amounts of methanol increase the methoxy ions on the surface of the catalyst [29]. Increased numbers of methoxy ions push the equilibrium toward FAME production [30]. A further increase in the methanol/oil ratio will dissolve most of the glycerol, which inhibits the reaction between methanol and the other reactants and the catalyst, thereby interfering with glycerin separation and product reduction [31].
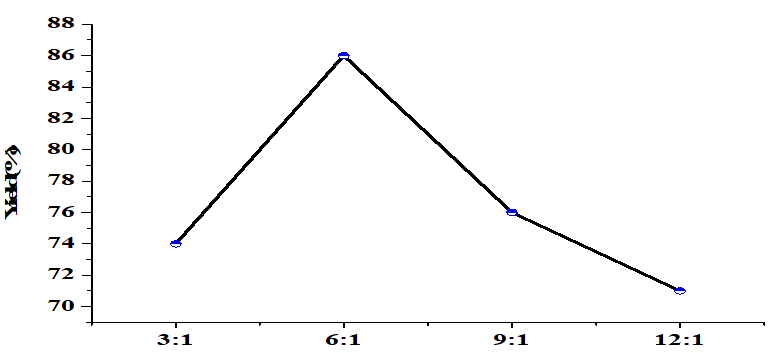
Figure 5. Effect of the methanol to oil molar ratio
Reaction time
The reaction time also significantly influences the transesterification yield when carried out under constant conditions (5 wt% catalyst, alcohol/oil molar ratio of 6: 1, 65oC). Figure 6 shows that yield increases with reaction time up until 4 hours with the optimal yield being 86%. Longer reaction times reduce yield. Optimal reaction time is reached when there is time for sufficient reactant interactions to produce the final product [32]. A shorter time means fewer reactant interactions and lower yield [25]. Conversely, a time longer than the optimal point causes a back reaction to occur and results in the formation of soap/saponification, which can reduce the amount of product [28].
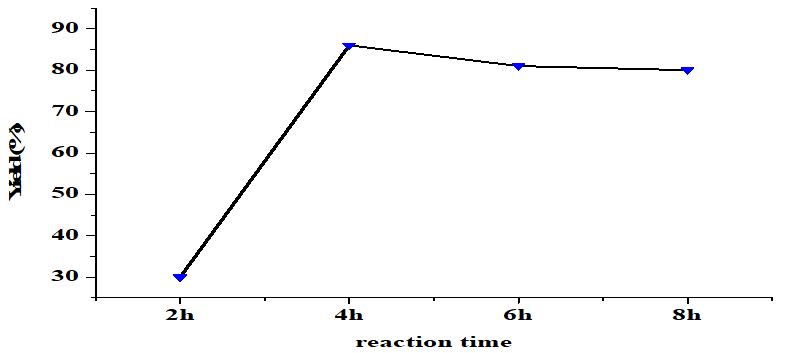
Figure 6. Effect of reaction time on the yield of FAME
Temperature
The effect of temperature on the transesterification process under fixed conditions (5wt% catalyst, the molar ratio of alcohol: oil 6: 1, 4h) is shown in Figure 7. Increasing the temperature increased yield up until 65oC with the optimal yield being 86%. Higher temperatures reduce yield. FAME production is an endothermic reaction that requires high temperatures [33]. The reaction rate is slow at low temperatures due to diffusion resistance; Heterogeneous catalysts form three phases producing an oil-methanol catalyst system [26]. Increasing the reaction temperature beyond the optimal conditions will cause the evaporation of methanol, which encourages saponification so that FAME production is reduced [34].
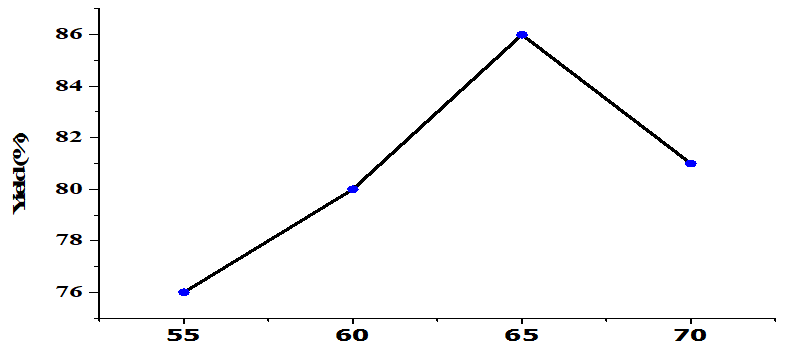
Figure 7. Effect of Reaction Temperature on yield
Reusability
After the reaction for 4h, the catalyst was separated from the mixture by filtration and washed using methanol and n-hexane [29] and dried at 110°C for reuse without prior recalcination. The FAME yield obtained from the reused catalyst for each reaction cycle shown in (Figure 8). The catalyst's effectiveness gradually decreases with repeated use. Catalytic activity is reduced due to the washing process [28]. Besides, the formation of Calcium diglyceroxide Ca (C3H7O3)2 as indicated by the XRD pattern will result in some saponification [23] which causes difficulties in the separation process, reducing catalyst recovery [35]. According to the SEM image illustrates the agglomeration of particles with other reactant molecules or catalyst leaching. This causes the loss of active sites, resulting in a reduced product yield [36].
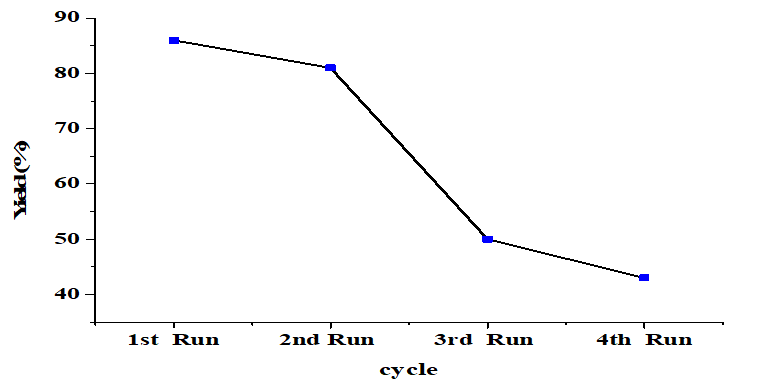
Figure 8. Reusability of the CaO catalyst
CONCLUSION
Lintau Bou limestone has high calcium content and can be used to produce a low-cost heterogeneous base CaO catalyst. Calcination (900°C for 5 hours) produces CaO particles which can be used in transesterification. Optimization of parameters indicated that the maximum yield of FAME obtained is 86%, which can be achieved using 5% by weight catalyst, 6: 1 ratio of methanol to oil, 4 hour reaction time, and a temperature of 65oC. The CaO catalyst could be reused three times indicating good reusability.
ACKNOWLEDGMENTS
The authors thank the Ministry of Research, Technology, and Higher Education, Republic of Indonesia, since part of this work is supported by DP2M DIKTI under the 2018 Pasca Sarjana Research Grant.
REFERENCES
- Maneerung T, Kawi S, Wang CH. Biomass gasification bottom ash as a source of CaO catalyst for biodiesel production via transesterification of palm oil. Energy Convers and Management. 2015;92, 234–43. DOI: 10.1016/j.enconman.2014.12.057.
- Al Makishah NH. Bioenergy: Microbial Biofuel Production Advancement. Int. J. Pharm. Res. Allied Sci. 2017;6(3):93-106.
- Beheshti M, Tahani A. Optimal Design Of Energy Sources In A Building Connected To Grid In The Presence Of Electric Vehicles. World Journal of Environmental Biosciences. 2018;3(S2):1-1
- Srivastava N, Kumar G. Biodiesel and its Production: Renewable Source of Energy. J. Biochem. Technol. 2019;10(3):1-10.
- Mansir N and Hin T-YY. Synthesis and characterization of solid heterogeneous catalyst for the production of biodiesel from high FFA waste cooking oil. Bayero Journal of Pure and Applied Sciences. 2018;10(1),62-66. DOI: 10.4314/bajopas.v10i1.13S.
- Rita Olivia, Novesar Jamarun, Syukri Arif, and Yenny Aydiyon Sirin. The Utilization Of Dolomite As Catalyst In Biodiesel Production. Rasayan Journal Chem. 2017;10(1), 160-164, DOI: 10.7324/RJC.2017.1011555.
- Kamisah D. Padiangan, Novesar Jamarun, Syukri Arief, Wasinton Simanjuntak. Transesterification of Castor oil using MgO/SiO2 Catalyst and Coconut oil as Co-reactant. Oriental Journal Of Chemistry. 2016;32(1),385-390. DOI: 10.13005/ojc/320143.
- Sirisomboonchai S, Abuduwayiti M, Guan G, Samart C, Abliz S, Hao X. Biodiesel production from waste cooking oil using calcined scallop shell as catalyst. Energy Conversion And Management. 2015;95,242–247. DOI: 10.1016/j.enconman.2015.02.044.
- Wen Z, Yu X, Tu ST, Yan J, Dahlquist E. Biodiesel production from waste cooking oil catalyzed by TiO2 -MgO mixed oxides. Bioresource Technology. 2010;101(24), 9570–9576. DOI:10.1016/j.biortech.2010.07.066.
- Mohamad M, Ngadi N, Wong S, Yahya NY, Inuwa IM, Lani NS. Synthesis and characterization of CaO-TiO2 for transesterification of vegetable palm oil. International Journal of Engineering Transactions B Applications. 2018;31(8),1326–1333. DOI: 5829/ije.2018.31.08b.22.
- Teo SH, Rashid U, Taufiq-Yap YH. Biodiesel production from crude Jatropha Curcas oil using calcium-based mixed oxide catalysts. Fuel. 2014;136, 244–252. DOI: 10.1016/j.fuel.2014.07.062.
- Farooq M, Ramli A, Naeem A, Mahmood T, Ahmad S, Humayun M. Biodiesel production from date seed oil (Phoenix dactylifera L.) via eggshell derived heterogeneous catalyst. Chemical Engineering Research And Design. 2018;132, 644–654, DOI:10.1016/j.cherd.2018.02.002.
- Linggawati A, Preparation and Characterization of Calcium Oxide Heterogeneous Catalyst Derived from Anadara Granosa Shell for Biodiesel Synthesis In Proceedings of, Conference on Instrumentation, Environment and Renewable Energy, UNRI Pekanbaru, pp. 1–8(2016), DOI: 10.18502/keg.v1i1.494.
- Sudsakorn K, Saiwuttikul S, Palitsakun S, Seubsai A. Limtrakul J. Biodiesel production from Jatropha Curcas oil using strontium-doped CaO/MgO catalyst. Journal of Environmental Chemical Engineering. 2017;5(3),2845–2852. DOI:10.1016/j.jece.2017.05.033.
- Gimbun J, Ali S, Charan Kanwal CCS, Shah LA, Ghazali NHM, Cheng CK. Biodiesel Production from Rubber Seed Oil Using A Limestone Based Catalyst. Advances in Materials Physics and Chemistry. 2012;02(04),138–141(2012). DOI: 10.4236/ampc.2012.24b036.
- Anjana PA, Niju S, Meera Sheriffa Begum KM, Anantharaman N. Utilization of Limestone Derived Calcium Oxide for Biodiesel Production From Non-Edible Pongamia Oil. Environmental Progress and Sustainable Energy. 2016;00(00),1–7. DOI: 10.1002/ep.
- Mansir N, Teo SH, Rashid U, Saiman MI, Tan YP, Alsultan GA. Modified waste eggshell derived bifunctional catalyst for biodiesel production from high FFA waste cooking oil. A review. Renewable and Sustainable Energy Reviews. 2018;82, 3645–3655. DOI: 10.1016/j.rser.2010.098.
- Mahreni, Sulistyawati. Utilization of Egg Shells as a Palm Oil and Methanol Biodiesel Catalyst, 2011: 1–6.
- Sumarlin, La Ode. Quality Analysis of Used Cooking Oil for Rejuvenation Using Natural and Calcined Diatomite Soils, 2008:4 8-10.
- Boro J, Thakur AJ, Deka D. Solid oxide derived from waste shells of Turbonilla striatula as a renewable catalyst for biodiesel production. Fuel Processing Technology. 2011;92(10), 2061–2067. DOI: 10.1016/j.fuproc.2011.06.008.
- Granados ML, Alonso DM, Sádaba I, Mariscal R, Ocón P. Leaching and homogeneous contribution in liquid phase reaction catalyzed by solids: The case of triglycerides methanolysis using CaO. Applied Catalysis B: Environmental. 2009;89, 265–272, DOI: 10.1016/j.apcatb.2009.02.014.
- Kouzu M, Kasuno T, Tajika M, Yamanaka S, Hidaka J. Active phase of calcium oxide used as solid base catalyst for transesterification of soybean oil with refluxing methanol. Applied Catalysis A: General. 2008;334,357–365. DOI: 10.1016/j.apcata.2007.10.023.
- Kawashima A, Matsubara K, Honda K. Acceleration of catalytic activity of calcium oxide for biodiesel production. Bioresource Technology. 2009;100(2),696–700. DOI: 10.1016/j.biortech.2008.06.049.
- Mootabadi H, Salamatinia B, Bhatia S, Abdullah AZ. Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel. 2010;89(8),1818–1825. DOI: 10.1016/j.fuel.2009.12.023.
- Konwar LJ, Boro J, Deka D. Activated Carbon Supported CaO from Waste Shells as a Catalyst for Biodiesel Production. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects. 2018;40(6),601–607. DOI: 10.1080/15567036.2012.733483.
- Kamisah D. Pandiangan, Wasinton Simanjuntak, Ilim, Heri Satria, and Novesar Jamarun. Catalytic Performance of CaO/SiO2 Prepared from Local Limestone Industry and Rice Husk Silica. Journal of pure Pure and Applied. Chemistry.Research. 2019; 8(2), 170-178. DOI: 10.21776/ub.jpacr.2019.008.002.459.
- Jitputti J, Kitiyanan B, Rangsunvigit P, Bunyakiat K, Attanatho L, Jenvanitpanjakul P. Transesterification of crude palm kernel oil and crude coconut oil by different solid catalysts. Chemical Engineering Journal. 2006;116(1),61–66. DOI: 10.1016/j.cej.2005.09.025.
- Reddy ANR, Saleh AA, Islam MS, Hamdan S. Active Razor Shell CaO Catalyst Synthesis for Jatropha Methyl Ester Production via Optimized Two-Step Transesterification. Journal of Chemistry. 2017;2017(1),1-20. DOI:10.1155/2017/1489218.
- Buasri A, Chaiyut N, Loryuenyong V, Wongweang C, Khamsrisuk S. Application of Eggshell Wastes as a Heterogeneous Catalyst for Biodiesel Production. Sustainable Energy. 2013;1(2), 7–13. DOI: 10.12691/rse-1-2-1.
- Obadiah A, Swaroopa GA, Kumar SV, Jeganathan KR, Ramasubbu A. Biodiesel production from Palm oil using calcined waste animal bone as catalyst. Bioresource Technology. 2012;116, 512–516. DOI: 10.1016/j.biortech.2012.03.112.
- Viriya-Empikul N, Krasae P, Puttasawat B, Yoosuk B, Chollacoop N, Faungnawakij K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresource Technology. 2010; 101(10), 3765–3767, DOI: 10.1016/j.biortech.2009.12.079.
- Garnica JAG, De Lima Da Silva N, Maciel MRW. Production and purification of biodiesel and glycerine, since vegetal oils and kinetic of vegetal oils transesterification reaction for wasted frying oil. Chemical Engineering Transaction. 2009;17, 433–438. DOI: 10.3303/CET0917073.
- Alves CT, Oliveira A, Carneiro SAV, Silva AG, Andrade HMC, Vieira De Melo SAB. Transesterification of waste frying oil using a zinc aluminate catalyst,” Fuel Process Fuel Processing Technology. 2013;106,102–107. DOI: 10.1016/j.fuproc.2012.07.008.
- Lee H V., Juan JC, Taufiq-Yap YH, Kong PS, Rahman NA. Advancement in heterogeneous base-catalyzed technology: An efficient production of biodiesel fuels. Renewable And Sustainable Energy. 2015;7(3), 1-46. DOI: 10.1063/1.4919082.
- Taufiq-Yap YH, Lee H V., Hussein MZ, Yunus R. Calcium-based mixed oxide catalysts for methanolysis of Jatropha curcas oil to biodiesel. Biomass and Bioenergy. 2011; 35(2), 827–834, DOI: 10.1016/j.biombioe.2010.11.011.
- Kaur, M., Ali, A. Lithium-ion impregnated calcium oxide as nanocatalyst for the biodiesel production from Karanja and jatropha oils. Renewable Energy. 2011; 81, 421–431. DOI: 10.1016/j.renene.2011.04.014.
