Archive \ Volume.11 2020 Issue 2
Metabolic syndrome and chronic diseases in Iran;
A case - control meta-analysis
Khadijeh Kalan Farmanfarma1, Mahmoud Ali Kaykhaei1, Mehdi Mohammadi1, Hosein Ali Adineh2, Alireza Ansari-Moghaddam1*
1 Department of Epidemiology & Biostatistics, Health Promotion Research Center, Zahedan University of Medical Sciences, Zahedan, Iran. 2 Department of Epidemiology & biostatistics, School of health, Iranshahr University of Medical Sciences, Iranshahr, Iran.

Abstract
Background: To quantify the frequency of metabolic syndrome amongst individuals suffering from some chronic diseases and to compare them with the data obtained from healthy population. Methods: Manuscripts published on the prevalence of metabolic syndrome between 2000 and 2016 were identified through the following databases; Magiran, SID, and Iran Medex as well as PUBMED, EMBASE, MEDLINE, Web of Science, Scopus, and Google scholar using related MESH terms. Studies included if they had published quantitative estimates and measure of variability and/or confidence limits in the individual with the following diseases: diabetes, cardiovascular disease, cancer, periodontal, arthritis, polycystic ovary syndrome, Non-alcoholic fatty liver, psychiatric disorder, kidney and chronic disease. Data were analyzed with Stata, version 11. Results: A total of 62 articles selected for the final stage of this meta-analysis. The pooled prevalence of metabolic syndrome among diabetes, those with cardiovascular diseases, cases with renal failure, patients with NAFLD, periodontal subjects and individuals by arthritis were significantly higher than healthy population (P < 0.05). The risk of aforementioned diseases was found to be two to six times higher in those with metabolic syndrome than the normal population. Comparably, there was an inverse association between metabolic syndrome and mental disorders. Conclusions: Our findings suggest the early recognition, control and prevention of the metabolic syndrome and its individual components in the general population.
Keywords: Metabolic Syndrome, Prevalence, Chronic disease

INTRODUCTION
Metabolic syndrome (MetS) is a set of metabolic disorders including central obesity, insulin resistance or glucose absorption and metabolism disorder, lipid disorders, and hypertension. [1] There are several criteria for diagnosing metabolic syndrome based on which a person having three of the above-mentioned factors is considered as having metabolic syndrome. [2]According to the available statistics, 25% of the world population on average suffer from metabolic syndrome [3] which varied across different regions of the world. [1] Nevertheless, studies showed that the frequency of metabolic syndrome is higher in certain diseases compared to the normal population including diabetes, [3] cardiovascular diseases,[4] renal failure [5] and non-alcoholic fatty liver (NAFLD). [6]
Accordingly, studies estimated that individuals with metabolic syndromes have six-fold greater risk of diabetes compared with individuals without metabolic syndrome. [7] Data also suggests that Subjects with identified MetS have more than 3 times higher statistically significant probability to get cardiovascular and NAFLD diseases. [4, 8] Moreover, the available evidence reported more than 30% increased risk for periodontal disease, chronic renal failure and colorectal cancer [9-11] incidence amongst people suffering from MetS than those without MetS. There has been some association between mental disorders and MetS as well. [12] In comparison, contradictory results are reported for some diseases such as arthritis (inverse association) in which the frequency of metabolic syndrome is lower compared to healthy population. [13]
On the other hand, no study has quantified the pooled prevalence of MS amongst unhealthy population of Iran systematically. Therefore, the purpose of the current study was two folds; firstly, to estimate the frequency of metabolic syndrome within these individuals and secondly, to compare it with the healthy population through a systematic review and meta-analysis. To our knowledge, this is the first case-control study in which representative and comparable sample of cases and controls selected systematically from the general and unhealthy population of different areas of a nation.
METHODS
Search strategy & Data sources:
Relevant published articles in English or Persian between 2000 to 2016 were identified through national databases (manuscripts published in Persian i.e. Magiran, SID, and Iran Medex) as well as PUBMED, EMBASE, MEDLINE, Web of Science, Scopus, and Google Scholar databases using MOOSE guidelines and MESH heading search strategy with the terms metabolic syndrome, diabetes, cardiovascular disease, cancer, periodontal, arthritis, polycystic ovary syndrome, Non-alcoholic fatty liver, psychiatric disorder, kidney and chronic disease. Additionally, references from identified manuscripts also scanned to find out any other relevant articles.
Study selection and Data extraction:
All cross-sectional and cohort studies on the Iranian population included if they used the three criteria of standard measurement, including NCEP-ATPIII, IDF, and ATPIII to estimate the prevalence of metabolic syndrome. Studies were also included if they had published quantitative estimates and measure of variability and/or confidence limits. Articles were excluded if they reported only one estimate of frequency, with no information by which to estimate the confidence intervals. In addition, in case of duplicate articles, more recent ones or those with a larger sample size were included. In the case of studies lacking sufficient information, researchers contacted the corresponding authors via telephone or email address in some cases.
Using this approach, a total of 62 articles determined for the final stage of the meta-analysis. The full text of selected articles were reviewed independently by two researchers and information was extracted and recorded in a checklist in Excel including: name of the author, year of publication, location of study, age and sex of participants, sample size, criterion for measuring metabolic syndrome, the general prevalence of metabolic syndrome and its confidence interval (CI).
Data synthesis and statistical analysis:
Pooled estimates and their 95% confidence intervals (CI) obtained for the prevalence of MS by means of a ‘random effects’ method and each report weighted according to an estimate of its ‘statistical size’ defined as the inverse of the variance of the log MS. Heterogeneity was estimated using the I2 statistic and tested using the Q statistic. To maintain consistency across studies, the CI for all reports calculated using the exact method in Stata. Figures obtained from the published systematic review [14] on the prevalence of MS among healthy population of Iran in the same period of time used as the control group to estimate the odds ratio (OR) and CI. Data analysis implemented using Stata, version 11.
RESULTS
The initial search of electronic databases produced 1313 records during time period of 2000 to 2016, of which 1193 were irrelevant. Twenty- seven were excluded from this meta-analysis as did not met inclusion criteria and 31 additional studies contained duplicate information. Then, a total 62 manuscripts with information on 22350 individuals with the published estimates of the prevalence of MetS among unhealthy population of Iran were eligible for inclusion in these analyses. The summary characteristics of selected studies, including authors, year of publication, province, place of residence, sample size, sex, age, criterion of measurement, as well as prevalence and 95% CI for MetS are shown in Table 1.
The pooled prevalence of reports on metabolic syndrome amongst unhealthy population of Iran are shown in Table 2. Consequently, Figure 1 displays the link between Mets and some chronic diseases in Iran through Odds ratios which obtained from the comparison of aforementioned frequencies with the combined prevalence of MetS in healthy population of Iran [14]. These figures and relationships are described in more detail in the following sections.
Diabetes and metabolic syndrome
A total of 21 reports with information on 9615 individuals with diabetes were included in these analyses. The pooled prevalence of metabolic syndrome among diabetes was 74.07% (95% CI: 69.39 - 79.07). Individuals with MetS were 6.54 times more likely to get diabetes compared with unaffected ones: OR = 6.54 (95% CI: 6.24 - 6.85).
Cardiovascular diseases and metabolic syndrome
From the identified studies, a total of 16 reports with information on 6211 subjects with cardiovascular diseases met inclusion criteria for these analyses. The summary estimates of metabolic syndrome in patients with cardiovascular disease was 46.58% (95% CI: 41.00 - 52.92). The risk of developing cardiovascular disease was approximately two times greater in persons who were categorized as metabolic syndrome compared with those classed as health/normal population: OR = 1.99 (95% CI: 1.89 - 2.10).
Renal failure and metabolic syndrome
Overall, 8 of the included studies identified frequency of metabolic syndrome in 922 cases with renal failure in which the combined estimate was 57.03% (95% CI: 49.52 - 65.67). The probability of renal failure’s occurrence was three-fold higher in MetS patients than normal counterparts; OR = 3.04 (95% CI: 2.66 - 3.46).
Non-alcoholic fatty liver and metabolic syndrome
Only, one study examined the prevalence of metabolic syndrome in sample of 864 patients with NAFLD. The frequency of metabolic syndrome in cases with Non-alcoholic fatty liver was estimated 65.03% (95% CI: 61.85 - 68.38). There was a significant association between metabolic syndrome and occurrence of NAFLD; OR = 4.26 (95% CI: 3.70 - 4.90).
Periodontal disease and metabolic syndrome
Reviewing literature ascertained one report (n = 278) in which 53.6% (95% CI: 47.54 – 59.57) of periodontal subjects were suffering from MetS. The study also demonstrated a significant relationship between metabolic syndrome and the occurrence of periodontal disease; OR = 2.64 (95% CI: 2.08 - 3.34).
Arthritis and metabolic syndrome
The pooled prevalence of MetS was 46.81% (95% CI: 42.53 - 51.53) in a sample of 3 reports including 523 individuals by arthritis. There has been a significant association between metabolic syndrome and arthritis [OR = 2.01; 95% CI: 1.69 - 2.39] in the present study. In other words, compared to the healthy population, those with MetS were at two-fold increased risk of developing arthritis.
Mental disorders and metabolic syndrome
Ten studies examined frequency of MetS in a total of 1495 people with mental disorders. The summary estimate of this study was 25.75% (95% CI: 20.60 - 32.19). Accordingly, an inverse association was observed between metabolic syndrome and mental disorders; OR=0.79 (95% CI: 0.70 -0.89).
Polycystic Ovary syndrome (PCOS) and metabolic syndrome
From the studies identified in the preliminary search, and a total of 9 studies involving 2242 subjects with PCOS were included. The combined frequency of MetS in patients with PCOS was found to be 29.06% (95% CI: 23.16 - 36.46). The risk estimate (OR) of metabolic syndrome among PCOS individuals was 0.93 (95% CI: 0.85 - 1.02) compared to healthy controls.
Colorectal cancer and metabolic syndrome
There was one study with a sample size of 200 patients with colorectal cancer in which frequency of metabolic syndrome was found to be 36% (95% CI: 29.35 - 43.07). The association between metabolic syndrome and colorectal cancer was not statistically significant [OR=1.28; 95% CI: 0.96-1.71] in this analysis.
Heterogeneity
There was no evidence of heterogeneity (P > 0.05) across the pooled prevalence’s obtained using three different diagnostic criteria for each disease except for kidney (P = 0.012). Therefore, a subgroup analysis was carried out to show the association between metabolic syndrome and renal failure using various diagnostic criteria. The prevalence varied from 48.2 (95% CI: 39.90 – 56.71) in IDF to 65.57 (95% CI: 57.17 - 75.19) in NCEP-ATPIII as shown in Table 2. Consequently, the strength of the association also differed according to the definition of MetS used which were as follow: IDF [OR = 2.13; 95% CI: 1.54 - 2.90]; ATPIII [OR = 2.20; 95% CI: 1.79 - 2.70]; NCEP-ATP III [OR = 4.35; 95% CI: 3.65 - 5.18].
|
Table 1: Prevalence of metabolic syndrome among unhealthy population of Iran by study. |
|||||
|
Metabolic syndrome |
Sample |
Setting |
First Author/ Year (Reference Number) |
||
|
P (95% CI) |
Diagnostic criteria |
Age |
No. (Sex) |
Province(District) |
|
|
17.4 (14.1 - 21.0) |
ATPIII |
58.01 ± 10.43 |
495 (MF) |
Tehran (Tehran) |
Mohagheghi, 2011 [15] |
|
59.1 (55.6 - 62.3) 51.4 (47.9 - 54.8) |
ATPIII IDF |
≥ 30 |
840 (MF) |
Tehran (Tehran) |
Hadaegh, 2008 [16] |
|
41.7 (26.3 – 41.3) 41.3 (23.5 – 38.1) |
IDF ATPIII |
<50 - ≥60 |
309 (MF) |
Khorasan (Mashhad) |
Assali, 2011 [17] |
|
48 (40.9 - 55.1) |
ATPIII |
70.7 ± 8.6 |
200 (MF) |
Kurdistan (Saqqez)
|
Ghanei –Gheshlagh , 2016 [18] |
|
77 (71.1 - 82.2( |
ATPIII |
59 ± 9.3 |
235 (MF) |
Tehran (Tehran) |
Ardeshiri, 2014 [19] |
|
39.7 (35.5 – 44.0) |
NCEP-ATPIII |
58.95 ± 9.91 |
531 (MF) |
Tehran (Tehran) |
Anvari , 2009 [20] |
|
59.8 (55.8 - 63.6) |
NCEP-ATPIII |
45.25 ± 6.40 |
637 (MF) |
Tehran (Tehran) |
Sadeghian, 2007 [21] |
|
78.7 (69.7 - 85.9) |
IDF |
58.25 ± 9.83 |
108 (MF) |
Yazd (Yazd) |
Mahdavi Anari,2015 [22] |
|
51 (46.8 - 55.2) 56 (47.4 – 64.3) |
ATPIII |
≥ 35 |
564 (MF) 141 (MF) |
Isfahan (Najafabad &Arak Isfahan) |
Harandi, 2016 [23] |
|
42.8 (38.5 - 47.0) |
NCEP-ATPIII |
57.1 ± .6 |
547 (MF) |
Isfahan (Isfahan) |
Kabir, 2012 [24] |
|
23.1 (17.8 – 29.0) 46.2 (37.5 – 55.0) |
IDF |
18 - 75 |
234 (M) 132 (F) |
Khorasan (Mashhad)
|
Parizadeh, 2014 [25] |
|
62.2 (56.7 – 67.4) |
NCEP-ATPIII |
60 ± 12.5 |
331 (MF) |
West-Azerbaijan (Urmia)
|
Dehghani, 2016 [26] |
|
74.4 (63.2 – 83.5) |
ATPIII |
< 55 |
78 (F) |
Isfahan (Isfahan) |
Sarrafzadegann, 2009 [27] |
|
52.3 (38.0 – 67.8) 25.7 (18.7 – 33.1) 31.3 (25.4 - 38.1) 26.1 (21.7 – 30.5) |
NCEP-ATPIII |
45 ± 5 68 ± 9 |
47 (F) 153 (M) 219 (F) 404 (M) |
Yazd (Yazd) |
Sadrebafghi, 2005 [28] |
|
69.7 (57.1 - 80.4) |
ATPIII |
58.36 ± 11.46 |
66 (MF) |
Isfahan (Nagafabad) |
Saeidi, 2009 [29] |
|
88.3 (85.5 - 90.6) |
NCEP-ATPIII |
43.33 ± 0.47 and 60.35 ± 0.38 |
639 (F) |
Tehran (Tehran) |
Nakhjavani, 2014 [30] |
|
76.79 (71.5 - 81.5) 75.42 (70.0 - 80.2) |
IDF modified ATPIII |
53.11 ± 10.15 |
293 (MF) |
Golestan (Gorgan) |
Marjani, 2011[31] |
|
73.1 (68.1 - 77.7) 64.9 (59.6 - 69.8) |
NCEP-ATPIII IDF |
54.08 ± 10.53 |
350 (MF) |
khozestan (Ahvaz) |
Rashidi, 2012 [32] |
|
73.4 (70.4 - 76.1) 64.9 (61.8 - 67.9) |
NCEP-ATPIII IDF |
56.0 ± 11.6 |
950 (MF) |
kerman (kerman) |
Foroozanfar, 2015 [33] |
|
95.7 (78.0 - 99.8) 78.3 (56.2 - 92.5) |
IDF |
30 - 70 |
23 (MF) 23 (MF) |
Qazvin (Qazvin) |
Ziaee, 2012 [34] |
|
94.8 (93.5 - 95.9) |
ATPIII |
30 - 83 |
1392 (MF) |
kerman (Rafsanjan) |
Derakhshan, 2010 [35] |
|
89.5 (80.3 - 95.3 ) |
ATPIII |
59.7 ± 8.8 |
76 (MF) |
Tehran (Tehran) |
Hosseinpanah, 2006 [36] |
|
71 (68.6 - 73.2) |
ATPIII |
54.3 ± 11.2 |
1534 (MF) |
Tehran (Tehran) |
Arash, 2016 [37] |
|
48 ( 38.8 – 57.1) |
modified NCEP III |
21 - 75 |
123 (MF) |
Zanjan (Zanjan) |
Sharifi, 2013 [38] |
|
75.1 (71.1 – 78.7) |
NCEP-ATPIII |
20 - 60 |
514 (MF) |
khozestan (Ahvaz) |
Baeis, 2016 [39] |
|
84.1 (77.3 – 89.4) |
IDF |
28 - 75 |
157 (MF) |
khozestan (Ahvaz) |
Veissi, 2016 [40] |
|
90.4 (84.5 – 94.9) 76.6 (68.3 – 83.0) 87.2 (77.5 - 94.1) 69.8 (57.4 – 79.7) |
ATPIII IDF ATPIII IDF |
47.72 ± 5.72 47.06 ± 5.88 |
139 (MF) 72 (MF) |
Qom (Qom) |
Vafaeimanesh, 2016 [41] |
|
39.4 (31.0 – 48.2) |
ATPIII |
57.5 ± 1 |
132 (MF) |
Tehran (Tehran) |
NAKHJAVANI, 2011 [42] |
|
55.3 (49.3 – 61.2) |
NCEP-ATPIII |
30 - 70 |
282 (MF) |
Isfahan (Isfahan) |
Janghorbani, 2016 [43] |
|
69.6 (64.1 – 74.8) |
ATPIII |
30 - 64 |
300 (MF) |
Isfahan (Isfahan) |
Sbohani, 2016 [44] |
|
64.6 (62.4 – 66.6) |
NCEP-ATPIII |
52.47 ± 10.20 |
1962 (MF) |
Khorasan (Mashhad) |
Bonakdaran, 2009 [45] |
|
79.5 (75.9 – 82.6) 84.3 (81.1 - 87.1) |
IDF ATPIII |
54.2 ± 11.6 |
588 (MF) |
Tehran (Tehran) |
Azizi, 2007 [46] |
|
39.5 (32.6 - 46.6) |
ATPIII |
20 - 40 |
200 (F) |
Tabriz (Tabriz) |
Pourteymour, 2013 [47] |
|
22.7 (17.9 - 28.0) |
ATPIII |
15 - 40 |
282 (F) |
Tehran (Tehran) |
Moini, 2012 [48] |
|
13.5 (5.4 - 25.3) |
ATPIII |
24 ± 6.8 |
53 (F) |
khozestan (Ahvaz) |
Shahbazian, 2012 [49] |
|
24.9 (21.2 - 28.7) |
ATPIII |
18 - 42 |
539 (F) |
Isfahan (Isfahan) |
Mehrabian, 2011 [50] |
|
19.7 (16.6 - 23.0 ) |
NCEP-ATPIII |
28.6 ± 4.3 |
624 (F) |
Tehran (Tehran) |
Madani, 2016 [51] |
|
28.8 (22.8 – 35.3) |
ATPIII |
15 - 35 |
215 (F) |
Guilan (Rasht) |
Zahiri, 2016 [52] |
|
39.4 (30.1 – 48.6) |
NCEP-ATPIII |
16 - 45 |
115 (F) |
Khorasan (Mashhad) |
Layegh, 2016 [53] |
|
43.5 (23.1 – 65.5) 20 (9.0 - 35.6) |
ATPIII |
17 - 37 |
23 (F) 40 (F) |
Tabriz (Tabriz) |
Ebrahimi-Mamaghani, 2015 [54] |
|
46.4 (38.2 – 54.6) |
ATPIII |
16 - 48 |
151 (F) |
Tehran (Tehran) |
Moradi, 2009 [55] |
|
36 (29.3 - 43.0) |
ATPIII |
57.1 ± 13.9 |
200 (MF) |
Tehran (Tehran) |
Forootan, 2012 [56] |
|
39.8 ( 30.2- 49.9) |
NCEP-ATPIII |
27 - 75 |
103 (F) |
Khorasan (Mashhad) |
Saadatian, 2012 [57] |
|
38.7 (33.7 - 43.8 ) 27.4 (22.9 - 32.2 ) 37.6 ( 32.6 - 42.7) |
IDF ATPIII NCEP-ATPIII |
23 - 70 |
372 (MF) |
Tehran (Tehran) |
Rezaei, 2009 [58] |
|
9.5 (5.8 - 14.4) 10 (6.2 - 15.0) |
IDF NCEP-ATPIII |
> 18 |
200 (MF) |
Hormozgan (Bandar Abbas) |
Khalili, 2015 [59] |
|
30.4 ( 25.0 - 36.1) |
ATPIII |
20 - > 40 |
280 (MF) |
Kermanshah (Kermanshah) |
Shakeri, 2016 [60] |
|
20.6 ( 15.9 - 25.9 ) |
ATPIII |
18 - 73 |
267 (MF) |
Golestan (Gorgan) |
Kamkar, 2016 [12] |
|
15 (8.6 – 23.5) |
NCEP-ATPIII |
18 - 60 |
100 (MF) |
Hormozgan (Bandar Abbas) |
Moayedi, 2015 [61] |
|
48.5 (36.2 – 60.9) |
NCEP-ATPIII |
18 - 65 |
68 (MF) |
Kerman (Kerman) |
Goughari, 2015 [62] |
|
25.7 (17.6 – 35.1) |
NCEP-ATPIII |
18 - 74 |
105 (MF) |
Zanjan (Zanjan) |
Ghoreishi, 2016 [63] |
|
57.6 (48.6 - 66.1 |
ATPIII |
18 - 76 |
132 (MF) |
West Azerbaijan (Uromeah)
|
Ghaneei –gheshlagh, 2011[64] |
|
56.33 (47.7 – 64.6) |
ATPIII |
20 - > 70 |
142 (MF) |
Golestan (Gorgan) |
Marjani, 2013 [65] |
|
67 (59.4 - 74.0) |
NCEP-ATPIII |
> 20 |
170 (MF) |
Isfahan (Isfahan) |
Mortazavi, 2012 [66] |
|
31.8 (22.4 – 42.4) |
ATPIII |
54 ± 17.4 |
91 (MF) |
Tehran (Tehran) |
Razeghi, 2011[67] |
|
59.5 (51.2 - 67.3) |
NCEP-ATPIII |
58.3 |
153 (MF) |
Tehran (Tehran) |
Shahrokh, 2012 [5] |
|
57 (46.3 - 67.7) |
NCEP-ATPIII |
> 35 |
89 (MF) |
Khorramabad (Borujerd) |
Maleki, 2015 [68] |
|
48.2 (39.9 - 56.7) 77.2 (69.5 – 83.7) |
IDF NCEP-ATPIII |
≥ 18 |
145 (MF) |
Markazi (Arak) |
Edalat-Nejad, 2015 [69] |
|
65.9 (60.1 – 71.4 ) 64.6 (60.5 – 68.4 ) |
ATPIII |
≥ 18 |
285 (M) 579 (F) |
Fars (Kavar) |
Fattahi, 2016 [70] |
|
53.6 (47.5 - 59.5) |
NCEP-ATPIII |
15 - 75 |
278 (MF) |
Kerman (Kerman) |
Safavi 2015 [71] |
|
46.1 (40.5 – 51.7) |
ATPIII |
57.2 ± 9.4 |
323 (MF ) |
Zanjan (Zanjan) |
Tehrani, 2015 [72] |
|
45.2 (33.5 - 51.8) |
ATPIII |
45.49 ± 14.21 |
120 (MF) |
Khorasan (Mashhad) |
Goshayeshi, 2012 [73] |
|
51.3 (39.8 – 62.5) |
ATPIII |
> 20 |
80 (MF) |
Tehran (Tehran) |
Shirani, 2016 [74] |
Abbreviations: ATPIII, Third Adult Treatment Panel; IDF, International Diabetes Federation; NCEP-ATPIII, National Cholesterol Education Program—Third Adult Treatment Panel; MF, Male &Female.
|
Table 2: Prevalence of Metabolic syndrome among unhealthy population of Iran by type of disease. |
||||
|
Disease |
Diagnostic criteria |
No. of reports |
Prevalence of metabolic syndrome (95% CI) |
P for heterogeneity |
|
Psychiatric Disorders |
|
|
|
0.91 |
|
|
ATPIII |
3 |
26.19(21.39 –32.06) |
|
|
|
NCEP-ATPIII |
5 |
26.79 (18.54 – 38.72) |
|
|
|
IDF |
2 |
19.51 (4.92 – 77.25) |
|
|
|
Pooled |
10 |
25.75(20.60 – 32.19) |
|
|
Polycystic Ovary Syndrome |
|
|
|
0.85 |
|
|
ATPIII |
7 |
29.67 (23.27 – 37.84) |
|
|
|
NCEP-ATPIII |
2 |
27.69 (14.04 -54.63) |
|
|
|
Pooled |
9 |
29.06 (23.16 -36.46) |
|
|
Cancer (Colorectal) |
|
|
|
- |
|
|
ATPIII |
1 |
36 (29.35 – 43.07) |
|
|
Arthritis |
|
|
|
0.67 |
|
|
ATPIII |
3 |
46.81 (42.53 – 51.53) |
|
|
Cardiovascular |
|
|
|
0.23 |
|
|
ATPIII |
7 |
53.01 (42.92-65.47) |
|
|
|
NCEP-ATPIII |
5 |
40.72(32.77 -50.59) |
|
|
|
IDF |
4 |
45.40 (33.15 – 62.19) |
|
|
|
Pooled |
16 |
46.58(41.00 -52. 92) |
|
|
Periodontal |
|
|
|
- |
|
|
NCEP-ATPIII |
1 |
53.6 (47.54 - 59.57) |
|
|
Kidney |
|
|
|
0.012 |
|
|
ATPIII |
3 |
48.87 (37.41 – 63.83) |
|
|
|
NCEP-ATPIII |
4 |
65.57 (57.17 -75.19) |
|
|
|
IDF |
1 |
48.2 (39.90 – 56.71) |
|
|
|
Pooled |
8 |
57. 03 (49.52 – 65.67) |
|
|
Non-alcoholic fatty liver |
|
|
|
- |
|
|
ATPIII |
1 |
65.03 (61.85-68.38) |
|
|
Diabetes |
|
|
|
0.34 |
|
|
ATPIII |
6 |
76 .07 (68.21 -84.85) |
|
|
|
NCEP-ATPIII |
8 |
69.14 (62.15 – 76.91) |
|
|
|
IDF |
7 |
75.94 (69.90 -82.51) |
|
|
|
Pooled |
21 |
74.07 (69.39 -79.07) |
|
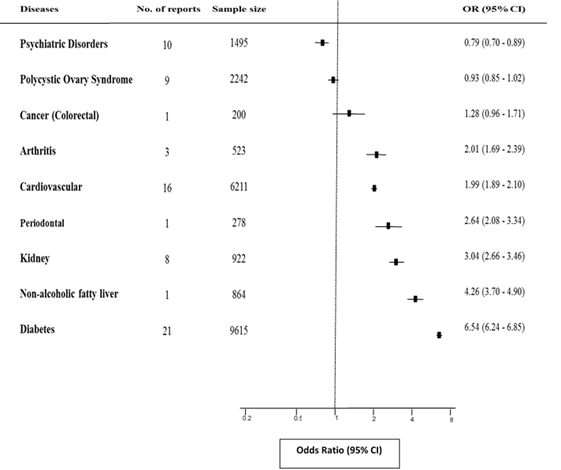
Figure 1. ORs and 95% CIs for metabolic syndrome comparing unhealthy (above-mentioned diseases-Cases) with healthy individuals of Iran (Controls). Black square, pooled point estimate; horizontal line, 95% CI for observed effect in each disease.
DISCUSSION
The synthesis of available articles in this systematic review and meta-analysis provides persuasive evidence of increased prevalence of metabolic syndrome among patients with type 2 diabetes, non-alcoholic fatty liver, renal failure, periodontal, cardiovascular disease and arthritis compared with general population. Accordingly, data suggests that the presence of MetS is a significant predictor of aforementioned diseases. In contrast, individuals with mental disorders had an overall decreased prevalence of metabolic syndrome than those of controls. Moreover, there were no evidence of association between metabolic syndrome with polycystic ovary syndrome and colorectal cancer in the present study.
In this study, the risk of diabetes was found to be six times higher in those with metabolic syndrome than the healthy population. The estimated odds ratio was in line with the findings from other studies in which the measure of association (RR) between the metabolic syndrome and incident of diabetes reported from 2 to 10. In the EPIC and the Framingham offspring studies, the hazard ratios were very close to the present study (4.59 and 6.90, respectively). [75] It seems that increased insulin resistance plays a significant role in the development of metabolic syndrome. [41] Moreover, impaired fasting glucose and abdominal obesity were the most significant component of MetS that predict incident of diabetes in these studies. [76] Nevertheless, the above-mentioned reports demonstrated that the absence of MetS strongly predict the development of diabetes regardless of other risk factors, including IGT and insulin resistance .[77]
Available data from epidemiologic studies supports the assumption that metabolic syndrome is a cluster of important etiologic factors for the development cardiovascular disease. [3] A meta-analysis of these reports revealed that the metabolic syndrome increase the risk of CVD and stroke by two-fold Similarly, this systematic review demonstrated that MetS is prevalent in approximately 47% patients with cardiovascular disease. Data also showed that patients with MetS have two times higher risk of developing CVD as well. Metabolic syndrome is the concurrent of multiple metabolic risk factors including IFG/IGT, obesity, hypertension, diabetes and dyslipidemia which any of them might predispose people to develop CVD. Then, there might be multiple mechanisms by which MetS could increase the risk of CVD.[78]
In the present review, approximately two-third of NAFLD patients were suffering from metabolic syndrome. In line with the previous reports, individuals with MetS were 4 times more likely to develop non-alcoholic fatty liver disease compared to those without MetS. [79] It seems that the increased level of ALT in patients with NAFLD is associated with the risk of metabolic syndrome. On the other hand, risk-factors of metabolic syndrome such as BMI, insulin resistance, abdominal obesity, increased blood glucose and TG, and reduced level of HDL-C are effective in increasing the level of ALT in those with NAFLD.[80] Furthermore, at least one of the MetS characters there might be presence amongst NAFLD cases.
A meta-analysis of 62 studies demonstrated that MetS and its individual traits independently increased the risk of chronic kidney disease. Likewise, this study strongly revealed a significant association between metabolic syndrome and renal failure. This might be explained by the fact that there is a relationship between the incidence of obesity [81] and hypertension [82] with renal failure. Moreover, in those with metabolic syndrome, urinary protein secretion is increased as a result of increased TG. Insulin resistance can also disrupt the function of mitochondrion and further damage the renal tissue cells by sodium retention, over-production of LDL cholesterol, and hypertriglyceridemia.[83]
The frequency of MetS in patients with arthritis in current study was about 46% which in accordance to some of previous studies [84] showed that presence of metabolic syndrome highly increased the risk of arthritis. Increased insulin resistance, [85] BMI, and subcutaneous and visceral fat in the first stages of arthritis [13] could possibly describe this relationship.
The available documents suggests a significant association between MetS and colorectal cancer. [86] The link between colorectal cancer and metabolic syndrome can be probably justified by the increased BMI, blood pressure and glucose [11] in these patients as well as the role of total cholesterol and TG in creating colon adenoma and differentiating colon polyps into cancerous types upon increasing insulin resistance. [87] Nevertheless, inconsistent with this data, there were no significant association between metabolic syndrome and colorectal cancer in this study. The existence of one report with small number of participants may explain this difference.
Another finding of the present study was a significant positive relationship between periodontal disease and metabolic syndrome. Then, the study supports the overall results of a meta-analysis, in which the existence of metabolic syndrome was linked to the occurrence of periodontal disease. [88] Some investigations have reported an association between periodontal diseases with specific components of MetS. Moreover, periodontal disease is one of the complications of diabetes and insulin resistance, which is in turn exacerbated by glucose disorder and incidence of diabetes. [89] These might be explanations for connection between two conditions.
The overall prevalence of metabolic syndrome among women with PCOS was found to be 29% in the present study that was not differed according to the definition used. Comparing to the healthy controls, no association was found between PCOS and the odds of being diagnosed with MetS. This contrasts the findings of a recently published meta-analysis in which individuals with PCOS were two times more likely to be diagnosed with MetS than individuals without PCOS. Principally, the combined frequency of MetS in this review was about 3% lower than our findings (26.30% vs. 29%) [90] Therefore, the reason for the paradox in reported risk may be differences in the reference group used for estimation of OR in two studies. For example, about one-third of general population (reference group) in Iran suffering from MetS [14] while occurrence of metabolic syndrome amongst general population is much lower in some reports.
Our findings suggest a lower prevalence of metabolic syndrome in patient with mental/psychological disorders compared to the healthy population which is in contrast to the other studies. [91] This difference may be due to the characteristics of patients, treatment methods, or the dosage of medications across studies. For example, the majority of patients were treated with first-generation antipsychotics drugs in the current study. In comparison, second generation drugs were used to treat these patients in other parts of the world. [59]
The current study is subject to several limitations. Firstly, there has been some methodological heterogeneity across studies. Secondly, the pooled ORs is obtained based on cross-sectional reports rather than on longitudinal data. Thirdly, there were inadequate data on confounders by which we were unable to adjust for some potential confounders. Nevertheless, there are some advantages which robust the findings and estimates. The main strength of the present study is its large pooled representative sample size from different areas of a nation that enabled us to find out vigorous estimation of frequency of diseases and their relationship with metabolic syndrome in Iran. In addition, in our knowledge this is the first meta-analysis with the nature of case-control examining the risk of some chronic diseases among patients with MetS in which both cases and controls are approximately proper representative sample of patients and general healthy population. Moreover, the sub-analysis conducted on different criteria used for classification of MetS yielded similar results and enhance our certainty regarding interpretation of results.
In conclusion, this meta-analysis supports the association between metabolic syndrome and some chronic diseases including diabetes, cardiovascular disease, renal failure, non-alcoholic fatty liver, periodontal, and arthritis. On the other hand, thes disease are now major global public health problems with high frequency and high morbidity that emphasize the early recognition, control and prevention of the metabolic syndrome and its individual components in the general population. Lifestyle interventions including healthy diet and physical activity can decrease the risk of metabolic syndrome to a great extent. Moreover, appropriate medical treatment of hyperglycemia, hypertension and dyslipidemia could be another important approach for the reduction of MetS consequences.
Funding
This manuscript is a part of Ph.D. thesis from Khadijeh Kalan FarmanFarma who has been funded by a scholarship from Zahedan University of Medical Sciences (Grant no.: 8140). Therefore, authors would like to express their appreciation to the Health Promotion Research Center and Zahedan University of Medical Sciences for their support
Authors’ contributions
The overall implementation of conception and study design, data collection including systematic review, critical appraisal of articles, extraction of information, synthesis, analysis and their interpretation as well as drafting and preparation of manuscript were the results of cooperative efforts by multiple individuals who has been listed as co-authors of this paper. All authors read and approved the final version of submitted article.
Conflict of Interest
There is no conflict of interest to be declared.
REFERENCES
- Xiao J, Wu CL, Gao YX, Wang SL, Wang L, Lu QY, et al. Prevalence of metabolic syndrome and its risk factors among rural adults in Nantong, China. Sci Rep. 2016; 6: 38089.
- Sarebanhassanabadi M, Mirhosseini SJ, Mirzaei M, Namayandeh SM, Soltani MH, Pedarzadeh A , et al. The incidence of metabolic syndrome and the most powerful components as predictors of metabolic syndrome in Central Iran: a 10-year follow-up in a cohort study. Iranian Red Crescent Med J. 2017; 19(7): e14934 .
- Neto L, Garcia JC, Xavier MDA, Borges JWP, Araújo MFMD, Damasceno MM, et al. Prevalence of metabolic syndrome in individuals with type 2 diabetes mellitus. Rev Bras Enferm. 2017; 70(2): 265-70 .
- Kim CJ, Park J, Kang SW. Prevalence of metabolic syndrome and cardiovascular risk level in a vulnerable population. Int J Nurs Pract. 2015; 21(2): 175-83.
- Shahrokh S, Heydarian P, Ahmadi F, et al. Association of inflammatory biomarkers with metabolic syndrome in hemodialysis patients. Ren Fail. 2012; 34(9): 1109-13.
- Gomes AR, de Sousa AW, de Miranda Henriques MS. Nonalcoholic Fatty Liver Disease in Metabolic Syndrome, Epidemiology and Pathogenesis. SmGroup. 2016; 1-15.
- Dragsbæk K, Neergaard JS, Laursen JM, Hansen HB, Christiansen C, Beck-Nielsen H, et al. Metabolic syndrome and subsequent risk of type 2 diabetes and cardiovascular disease in elderly women: challenging the current definition. Medicine. 2016; 95(36): e4806.
- Yang KC, Hung HF, Lu CW, Chang HH , Lee LT, Huang, KC. Association of non-alcoholic fatty liver disease with metabolic syndrome independently of central obesity and insulin resistance. Sci Rep. 2016; 6: 27034.
- Daudt LD, Musskopf ML, Mendez M, Remonti LLR, Leitão CB, Gross JL, et al . Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. Braz Oral Res. 2018; 32: e35.
- Alizadeh S, Ahmadi M, Ghorbani Nejad B, Djazayeri A, Shab-Bidar S. Metabolic syndrome and its components are associated with increased chronic kidney disease risk: Evidence from a meta‐analysis on 11 109 003 participants from 66 studies .Int J Clin Pract. 2018; 72: e13201.
- Alfa-Wali M, Boniface S, Sharma A, Tekkis P, Hackshaw A, Antoniou A. Metabolic syndrome (MetS) and risk of colorectal cancer (CRC): a systematic review and meta-analysis. World J Surg Med Radiat Oncol. 2015; 4(7): 41-52.
- Kamkar MZ, Sanagoo A, Zargarani F, Jouybari L, Marjani A. Metabolic syndrome in patients with severe mental illness in Gorgan. J Nat Sc Biol Med. 2016; 7(1): 62-7.
- Lee SH, Choi H, Cho BL, An AR, Seo YG, Jin HS. Relationship between metabolic syndrome and rheumatoid arthritis. Korean J Fam Med . 2016; 37(1): 44-50.
- Kalan Farmanfarma Kh, Kaykhaei MA, Adineh HA, Mohammadi M, Dabiri S, Ansari-Moghaddam A. Prevalence of metabolic syndrome in Iran: A meta-analysis of 69 studies. Diabetes Metab Syndr. 2019; 13(1): 792-99.
- Mohagheghi A, Panahi A, Hedayat DK, Ghorbani Yekta B. The effects of metabolic syndrome on left main coronary artery stenosis in coronary angiograms of patients: a two year study. TUMS. 2011; 69(9).571-75.
- Hadaegh F, Zabetian A, Tohidi M, Azizi F. Relationship between metabolic syndrome and coronary heart disease in Iranian population the Tehran lipid and glucose study. Tehran Univ Med J. 2008; 66 (8) : 590-98.
- Assali A, Ghayour-Mobarhan M, Sahebkar A, Hassani M, Kasaian J, Tatari F, et al. Association of angiotensin II type 1 receptor gene A1166C polymorphism with the presence of diabetes mellitus and metabolic syndrome in patients with documented coronary artery disease. Eur J Intern Med. 2011; 22(3): 254-61.
- Ghanei Gheshlagh R, Nourozi Tabrizi K, Shabani F, Zahednezhad H. Association between metabolic syndrome and sleep apnea in elderly patients with cardiovascular diseases. Med Sci J Islamic Azad Univ Tehran Med Branch. 2016; 26 (1): 46-51.
- Ardeshiri M, Faritus Z, Ojaghi-Haghighi Z, Bakhshandeh H, Kargar F, Aghili R. Impact of metabolic syndrome on mortality and morbidity after coronary artery bypass grafting surgery. Res Cardiovasc Med. 2014; 3(3): e20270.
- Anvari MS, Boroumand MA, Pourgholi L, Sheikhfathollahi M, Rouhzendeh M, Rabbani S, et al. Potential link of microalbuminuria with metabolic syndrome in patients undergoing coronary angiography. Arch Med Res. 2009; 40(5): 399-405.
- Sadeghian S, Darvish S, Salimi S, Esfehani FA, Fallah N, Mahmoodian M, et al. Metabolic syndrome: stronger association with coronary artery disease in young men in comparison with higher prevalence in young women. Coron Artery Dis. 2007; 18(3): 163-8.
- Mahdavi Anari L, Ghanbari-Firoozabadi M, Ansari Z, Emami M, Vafaii Nasab M, Nemaiande M ,et al. Effect of Cardiac Rehabilitation Program on Heart Rate Recovery in Coronary Heart Disease. J Teh Univ Heart Ctr. 2015; 10(4): 176-81.
- Harandi SA, Sarrafzadegan N, Sadeghi M, Talaei M, Dianatkhah M, Oveisgharan S, et al. Do cardiometabolic risk factors relative risks differ for the occurrence of ischemic heart disease and stroke?. ResCardiovasc Med. 2016; 5(1): e30619.
- Kabir A, Sarrafzadegan N, Amini A, Aryan RS, Kerahroodi FH, Rabiei K, et al. Impact of cardiac rehabilitation on metabolic syndrome in Iranian patients with coronary heart disease: the role of obesity. Rehabil Nurs. 2012; 37(2): 66-73.
- Parizadeh SA, Jamialahmadi K, Rooki H, Zaim-Kohan H, Mirhafez SR, Hosseini N, et al. Association of neuropeptide Y gene rs16147 polymorphism with metabolic syndrome in patients with documented coronary artery disease. Ann Hum Biol. 2015; 42(2): 179-83.
- Dehghani MR, Rezaei Y, Fakour S, Arjmand N. White blood cell count to mean platelet volume ratio is a prognostic factor in patients with non-ST elevation acute coronary syndrome with or without metabolic syndrome. Korean Circ J. 2016; 46(2): 229-38.
- Sarrafzadegann N, Ashrafi F, Noorbakhsh M, Haghighi M, Sadeghi M, Mazaheri F, et al. Association of breast artery calcification with coronary artery disease and carotid intima-media thickness in premenopausal women. East Mediterr Health J . 2009; 15(6): 1474-82.
- Sadr Bafghi S M, Rafiei M, Namayande S M, Andishmsnd A, Soltani MH, Motefaker M, et al. Examination of premature myocardial infarction in Yazd. TUMJ. 2005; 7: 579-89.
- Saeidi M. Metabolic Syndrome and Hypertension in Diabetic Patients. IJEM. 2009; 11(1): 11-16.
- Nakhjavani N, Imani M, Larry M, Aghajani-Nargesi A, Morteza A, Esteghamati A. Metabolic syndrome in premenopausal and postmenopausal women with type 2 diabetes: loss of protective effects of premenopausal status. J Diabetes Metab Disord. 2014; 13(1): 102.
- Marjani A, Shirafkan A. The metabolic syndrome in type 2 diabetic patients in Gorgan: According to NCEP ATPIII and IDF definitions. Diabetes Metab Syndr. 2011; 5(4): 207-10.
- Rashidi H, Fardad F, Ghaderian B, Shahbazian HB, Latifi M, Karandish M, et al. Prevalence ofMetabolic Syndrome and its Predicting Factors in Type 2 Diabetic Patients in Ahvaz. Jundishapur Sci Med J. 2012; 11(1): 163-75.
- Foroozanfar Z, Najafipour H, Khanjani N, Bahrampour A, Ebrahimi H . The Prevalence of Metabolic Syndrome According to Different Criteria and its Associated Factors in Type 2 Diabetic Patients in Kerman, Iran. Iran J Med Sci. 2015; 40(6): 522-25.
- Ziaee A, Hashemipoor S, Karimzadeh T, Jalalpoor A, Javadi A. Relation of Vitamin D3 Level with metabolic Syndrome Indices among Patients with Diabetes and Non-Diabetic Individuals. J Ardabil Univ Med Sci. 2012; 12(2): 149-56.
- Derakhshan R, Khoshnood A, Balaee P. Evaluation of Abdominal Obesity Prevalence in Diabetic Patients and Relation with Other Factors of Metabolic Syndrome. IJEM. 2010; 12(3): 208-12.
- Hosseinpanah F, Rambod M, Sadeghi L, Foroutan M, Naseri M. Assessing predicting factors in non-alcoholic fatty liver disease (NAFLD) in type 2 diabetes. Research in Medicine. 2006; 30 (1): 9-15.
- Nargesi AA, Esteghamati S, Heidari B, Hafezi-Nejad N, Sheikhbahaei S, Pajouhi A, et al. Nonlinear relation between pulse pressure and coronary heart disease in patients with type 2 diabetes or hypertension. J Hypertens. 2016; 34(5): 974-80.
- Sharifi F, Jaberi Y, Mirzamohammadi F, Mirzamohammadi H, Mousavinasab N. Determinants of developing diabetes mellitus and vascular complications in patients with impaired fasting glucose. Indian J Endocrinol Metab. 2013; 17(5): 899–905.
- Gholamzadeh Baeis M, Mohebi S, Miladinia M, Parham M. Study of Metabolic Syndrome Based on the NCEP/ATP III Criteria in People at Risk for Diabetes. Qom Univ Med Sci J. 2016; 10(6):42-50
- Veissi M, Anari R, Amani R, Shahbazian H, Latifi SM. Mediterranean diet and metabolic syndrome prevalence in type 2 diabetes patients in Ahvaz, southwest of Iran. Diabetes Metab Syndr . 2016; 10(2 Suppl 1): S26-29.
- Vafaeimanesh J, Bagherzadeh M, Mirzaei A, Parham M, Norouzinia M, Vafaee R . Effect of Helicobacter pylori on metabolic syndrome parameters in diabetic patients.Gastroenterol Hepatol Bed Bench. 2016; 9(Suppl1): S36–S
- Nakhjavani M, Esteghamati A, Tarafdari AM, Nikzamir A, Ashraf H, Abbasi M. Association of plasma leptin levels and insulin resistance in diabetic women: a cross-sectional analysis in an Iranian population with different results in men and women. Gynecol Endocrinol . 2011;27(1): 14-9.
- Janghorbani M, Amini M. Utility of Continuous Metabolic Syndrome Score in Assessing Risk of Type 2 Diabetes: The Isfahan Diabetes Prevention Study. Ann Nutr Metab. 2016;68(1): 19-25.
- Sobhani N, Entezari MH, Feizi A, Asgari GH, Reiesi J. Association of Dietary Fatty Acids with Metabolic Syndrome Indices in Patients with Type 2 Diabetes. J Isfahan Med Sch. 2016; 34(383): 563-71.
- Bonakdaran SH, Gharebaghi M, Vahedian M. Prevalance of Anemia in Type 2 Diabetic Patients and the Role of Nephropathy. Iranian Journal of Endocrinology & Metabolism. 2009 ;11(2): 127-34.
- Jahed A, Hosseinpanah F, Azizi F. Prevalence and predictive factors of lada “latent autoimmune diabetes in adults” in newly diagnosed diabetics of tehran lipid and glucose study. Ijdld. 2007; 7(1) : 43-54.
- Pourteymour Fard Tabrizi F, Alipoor B, Sadaghiani MM, Ostadrahimi A, Malek Mahdavi A. Metabolic syndrome and its characteristics among reproductive-aged women with polycystic ovary syndrome: A cross-sectional study in northwest Iran. Int J Fertil Steril. 2013; 6(4): 244–49.
- Moini A, Javanmard F, Eslami B, Aletaha N. Prevalence of metabolic syndrome in polycystic ovarian syndrome women in a hospital of Tehran. Iran J Reprod Med. 2012; 10(2): 127–30.
- Shahbazian HB, Shahbazian N, Haghighi M, Khodadadi M. Prevalence of Metabolic Syndrome in Patients with Poly Cystic Ovarian Syndrome in Ahvaz. Sci Med J. 2012; 10(6): 595-604.
- Mehrabian F, Khani B, Kelishadi R, Kermani N.The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females. J Res Med Sci. 2011; 16(6): 763–69.
- Madani T, Hosseini R, Ramezanali F, Khalili G, Jahangiri N, Ahmadi J, et al. Metabolic syndrome in infertile women with polycystic ovarian syndrome. Arch Endocrinol Metab. 2016; 60(3): 199-204.
- Zahiri Z, Sharami SH, Milani F, Mohammadi F, Kazemnejad E , Ebrahimi H ,et al. Metabolic syndrome in patients with polycystic ovary syndrome in Iran. Int J Fertil Steril. 2016; 9(4): 490–96.
- Layegh P, Mousavi Z, Tehrani DF, Parizadeh SM, Khajedaluee M. Insulin resistance and endocrine-metabolic abnormalities in polycystic ovarian syndrome: Comparison between obese and non-obese PCOS patients. Int J Reprod Biomed (Yazd). 2016; 14(4): 263–70.
- Ebrahimi-Mamaghani M, Saghafi-Asl M, Pirouzpanah S, Aliasgharzadeh A, Aliashrafi S, Rezayi N, et al. Association of insulin resistance with lipid profile, metabolic syndrome, and hormonal aberrations in overweight or obese women with polycystic ovary syndrome. J Health Popul Nutr. 2015; 33(1): 157–67.
- Moradi S, Darvishi N. Evaluation of the Prevalence of Metabolic Syndrome in Women with Polycystic Ovary Syndrome Referred to the Institute of Endocrine and Metabolism. RJMS. 2009; 16(63): 132-37.
- Forootan M, Tabatabaeefar M, Yahyaei M, Maghsoodi N. Metabolic syndrome and colorectal cancer: a cross-sectional survey. Asian Pacific J Cancer Prev. 2012; 13(10): 4999-5002.
- Saadatian V , Ghareh S , Shakeri MT, Emadzadeh M, Taraz Jamshidi SH, Emadzadeh A. The Frequency of Metabolic Syndrome among Female Patients Admitted in Psychiatry Ward . Medical Journal of Mashhad University of Medical Sciences. 2012; 54(4): 230-37.
- Rezaei O, Khodaie-Ardakani MR, Mandegar MH, Dogmehchi E, Goodarzynejad H. Prevalence of metabolic syndrome among an Iranian cohort of inpatients with schizophrenia. Int J Psychiatry Med. 2009; 39(4): 451-62.
- Khalili F, Moayedi F. Metabolic syndrome in schizophrenic patients: prevalence and findings in Bandar Abbas. Intl Res J.Appl BasicSci. 2015; 9(11): 1890-94.
- Shakeri J, Karimi K, Farnia V, Golshani S, Alikhani M. Prevalence of metabolic syndrome in patients with schizophrenia referred to farabi hospital, Kermanshah, Iran. Oman Med J. 2016; 31(4): 270–75.
- Moayedi F, Abdolrasouli LH, Fardan Y, Hosseini S, Sadeghi P. Prevalence of metabolic syndrome among bipolar patients. RJ PBCS. 2015; 6(2): 910-14.
- Goughari AS, Mazhari S, Pourrahimi AM, Sadeghi MM, Nakhaee N. Associations between components of metabolic syndrome and cognition in patients with schizophreni . J Psychiatr Pract. 2015; 21(3): 190-97.
- Ghoreishi A, Shajari Z, Sharifi F, Ghoreishi AB. Metabolic Syndrome, 10year–Coronary Heart Disease and 8year-Diabetes Mellitus Prediction in the Patients with Schizophrenia. Adv Biores. 2016; 7(4): 155-62.
- Ghanei Geshlagh R, Hemmati Maslakpak M , Ghoci S. Sleep apnea and metabolic syndrome in hemodialysis patients. Urmia Medical Journal. 2011 ; 22(4): 339-45.
- Marjani A, Moujerloo M, Hezarkhani S. Age related metabolic syndrome among hemodialysis patients in Gorgan, Iran. Open Biochem J. 2013; 7:15–8.
- Mortazavi M, Seirafian S, Naini AE, Zamani N, Moien N. The Prevalence of Metabolic Syndrome in Patients on Hemodialysis and Peritoneal Dialysis: A Comparative Study. JIMS. 2012; 29:171.
- Razeghi E, Heydarian P, Heydari M. The frequency of prediabetes and contributing factors in patients with chronic kidney disease. Rev Diabet Stud. 2011;8(2): 276- 81.
- Maleki A, Montazeri M, Rashidi N, Montazeri M, Yousefi-Abdolmaleki E. Metabolic syndrome and its components associated with chronic kidney disease. Journal of research in medical sciences: the official. J Res Med Sci. 2015; 20(5): 465–69.
- Edalat-Nejad M, Zameni F, Qlich-Khani M, Salehi F. Geriatric nutritional risk index: a mortality predictor in hemodialysis patients. Saudi J Kidney Dis Transpl. 2015; 26(2): 302-8.
- Fattahi MR, Niknam R, Safarpour A, Sepehrimanesh M, Lotfi M. The prevalence of metabolic syndrome in non-alcoholic fatty liver disease; a population-based study. Middle East J Dig Dis. 2016; 8(2): 131–37.
- Safavi F, Yousefzadeh GH, Shokoohi M, Safavi S, Najafipour H, Shadkam Farokhi M. Prevalence of metabolic syndrome in patients with periodontal disease. JKMU. 2015; 22(3): 229-39.
- Hossein Tehrani M, Karimi M, Kalhor L, Mazloumzade S. Prevalence of Metabolic Syndrome and its Components in Patients with Osteoarthritis. J Adv Med Biomed Res. 2015; 23(96): 68-77.
- Goshayeshi L, Saber H, Sahebari M, Rezaieyazdi Z, Rafatpanah H, Esmaily H ,et al. Association between metabolic syndrome, BMI, and serum vitamin D concentrations in rheumatoid arthritis.Clin Rheumatol. 2012; 31(8): 1197-203.
- Shirani F, Khaleghi S, Nikfam M, Pourmojarab A. The prevalence of metabolic syndrome in psoriatic arthritis patients, a hospital‐based cross-sectional study on Iranian population. TUMJ. 2016; 74(8): 569-77.
- Ford ES, Schulze MB, Pischon T, Bergmann MM, Joost HG, Boeing H. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam Study. Cardiovasc diabetol. 2008; 7(1): 35.
- Cheung BM, Wat NM, Man YB, Tam S, Thomas GN, Leung GM, et al. Development of diabetes in Chinese with the metabolic syndrome: a 6-year prospective study. Diabetes care . 2007; 30(6): 1430-36.
- Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes care. 2003; 26(11): 3153-59.
- Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P,et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 56(14): 1113-32.
- Salman Roughani H, Mohammadi S M, Arshadi L, Salman Roughani R, ZaremehrjerdiA. Investigating the Indices of Insulin Resistance and Metabolic Syndrome in Patients with Asymptomatic Fatty Liver. Govaresh. 2008 ; 13 (2): 75-
- Ghamar-Chehreh ME, Amini M, Khedmat H, Moayed Alavian S, Daraei F, Mohtashami R, et al. Elevated alanine aminotransferase activity is not associated with dyslipidemias, but related to insulin resistance and higher disease grades in non-diabetic non-alcoholic fatty liver diseaseAsian. Pac J Trop Biomed. 2012; 2(9): 702–6.
- Noori N, Hosseinpanah F, Nasiri AA, Azizi F. Comparison of overall obesity and abdominal adiposity in predicting chronic kidney disease incidence among adults. J Ren Nutr. 2009; 19(3): 228-37.
- Cho JA, Lee SJ, Reid EA, Jee SH. Metabolic syndrome component combinations and chronic kidney disease: the severance cohort study. Maturitas. 2013;75(1): 74-80.
- Raikou V, Gavriil S. Metabolic syndrome and chronic renal disease. Diseases. 2018; 6(1): 12.
- Naik M, Bhat T, Jalalie U, Ganayie M T, Waseem M, Bhat A. Prevalence and predictors of metabolic syndrome in rheumatoid arthritis. Int J Res Med Sci . 2017; 5(8): 3322-28.
- Lee SG, Kim JM, Lee SH, Kim KH, Kim JH, Yi JW, et al. Is the frequency of metabolic syndrome higher in South Korean women with rheumatoid arthritis than in healthy subjects? Korean J Intern Med. 2013; 28(2): 206 -15.
- Jinjuvadia R, Lohia P, Jinjuvadia C, Montoya S, Liangpunsakul S. The association between metabolic syndrome and colorectal neoplasm: systemic review and meta-analysis. J Clin Gastroenterol. 2013; 47(1): 33–44.
- Lim HS, Shin EJ, Yeom JW, Park YH, Kim SK . Association between nutrient intake and metabolic syndrome in patients with colorectal cancer. Clin Nutr Res. 2017; 6(1): 38-46.
- Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, et al. Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013; 98(3): 913-20.
- Kaye EK, Chen N, Cabral HJ, Vokonas P, Garcia RI. Metabolic syndrome and periodontal disease progression in men. J Dent Res. 2016; 95(7): 822-28.
- Hallajzadeh J, Khoramdad M, Karamzad N, Almasi-Hashiani A, Janati A, Ayubi E, et al. Metabolic syndrome and its components among women with polycystic ovary syndrome: a systematic review and meta-analysis. J Cardiovasc Thorac Res. 2018; 10(2): 56-69.
- Vancampfort D, Vansteelandt K, Correll CU Mitchell AJ, De Herdt A, Sienaert P, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013; 170(3): 265-74.
