Archive \ Volume.11 2020 Issue 4
Prevalence of Fungi in Human Follicular Fluid and Its Potential Impact on In Vitro Fertilization Process
Azhar Abdullah Najjar1,2*, Ebtesam Hamoud Alosaimi1, Hassan Salah Abduljabbar3, Howaida Abdulmonem Hashim3, Mohammed Abdulrahman Alem4, Mohamed Morsi M. Ahmed1,5, Samah Omar Noor 1
1 Department of Biological Sciences, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia, 2Microbiology Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. 3Department of Obstetrics and Gynecology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia. 4 Laboratory Directorate, Ministry of Health, Jeddah, Saudi Arabia.5 Nucleic Acids Research Dept., Genetic Engineering, and Biotechnology Research Institute (GEBRI), City for Scientific Research and Technological Applications. Alexandria, Egypt.
Abstract
Infertility is a significant interest as a global public health concern and is considered a big issue in Saudi society. Therefore, a lot of people choose to have in vitro fertilization (IVF) procedures to help women become pregnant. Follicular fluid (FF) is a potential source of microbes that may influence the results of the IVF procedure. Aim: The present study investigated the fungi isolated within 50 FF of women undergoing IVF procedure and the effect of these microbes on the IVF outcomes. Methodology: All yeast isolates were identified based on morphological characteristics, biochemical, and molecular analysis. Also, we determined the antimicrobial susceptibility against the yeast isolates. Results: Three Candida species belong to one genus were detected within 10% of FF samples and 90% did not show any fungal growth. The predominant species was Candida glabrata with (60%), followed by C. albicans (20%), and C. guilliermondii with (20%). All Candida species isolated showed the highest susceptibility against Micafungin and Voriconazole. There was no statistically significant difference in the IVF outcomes with the presence of microbes. Conclusion: Follicular fluid is not always sterile, but it contains different species of yeasts that are not correlated with the IVF outcomes and a larger sample is needed to further examine our hypothesis. Also, microbiological examination of FF in women who have undergone failed IVF cycles may provide an opportunity to initiate antimicrobial therapy before the next conception.
Keywords: Follicular fluid, In vitro fertilization, Oocyte retrieval, Fungi and Infertility
INTRODUCTION
Infertility is a significant interest as a global public health issue and is considered a major clinical problem today of different communities [1]. Women cannot get pregnant for at least a year of trying according to WHO definition [2]. The infertility prevalence was highest in some regions such as South Asia, North Africa, the Middle East, and Central Asia [3]. The total fertility rate of the births per woman in the world has decreased from 4.979 in 1960 to 2.432 in 2017 while the total fertility rate in Saudi Arabia has decreased from 7.30 in 1970–1975 to 3.03 in 2005–2010 [4, 5]. In 2012, the data of 457 infertility patients were collected and analyzed in Eastern Province, Saudi Arabia and they found that the infertility problem was 18.93%, which was higher than the prevalence in other countries 3.5% to 16.7% within one year [4]. There are several causes of female infertility such as endometrioses, polycystic ovary syndrome, endocrine disorders, genetic factors, tubal factors, and pelvic inflammatory disease [6]. Furthermore, lifestyle factors such as age, nutrition, obesity, smoking, environmental pollutants, and others can affect overall health and contribute to infertility problems [7]. Infertility is now recognized as a cause for interest in Saudi society. Therefore, a lot of people choose to have an assisted reproductive technology (ART) to help women become pregnant. One of these techniques is in vitro fertilization (IVF) procedure [8]. Prior to IVF cycles, couples are screened for the presence of sexually transmitted infections including Chlamydia trachomatis, gonorrhea, hepatitis B and C, syphilis, human immunodeficiency virus (HIV), and cytomegalovirus (CMV) but the routines of microbiological tests are not conducted [9]. One of the most difficult situations when embryos contamination occurs after spending a lot of money and time. A second potential negative result when the microorganisms are transmitted from the contaminated embryo culture media into the female reproductive system that can lead to negative pregnancy outcomes. Several cases of embryo contamination are caused by microorganisms from a follicular fluid (FF) [10]. It is a fluid that surrounds the ovum inside the ovary and plays an important role in the intercellular communication in the antral follicle while carrying nutrients to the oocyte. It is also an essential part of the success of natural fertilization found at every stage of the conception procedure [11, 12]. The FF can be colonized or contaminated by microorganisms [13]. Also, the embryo culture media contains antibiotics for bacteria while it does not routinely contain any antifungal agents, which is the cause of the common fungal contamination in IVF culture [10].
Previous studies were associated with the FF microorganisms and the IVF outcomes [9, 13-16]. As far as we know, in Saudi Arabia, no similar studies have been performed on this topic yet and due to the high number of infertile people who undergo the IVF cycle, this kind of study is important and will provide an important piece of information that will help doctors to improve the IVF procedure.
This study aims to identify the fungi in the FF of women who undergo IVF cycles at the time of oocyte retrieval and correlated these microorganisms with IVF outcomes.
We hypothesized that FF microorganisms may affect the success rate of the IVF cycles.
MATERIALS AND METHODS
Specimen collection
From October 2018 to April 2019, a cross-sectional study conducted for 50 FF specimens were collected from women undergoing IVF procedure at the time of oocyte retrieval. Ethics approval was obtained from the Biomedical Ethics Unit at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. All partners gave their written informed consent to participate in this study and permission to access their medical history.
The inclusion criteria are fertile women who have an infertile male partner (male factor), and women who have causes of infertility include (endometriosis, ovulatory disorders, tubal factor, and unexplained infertility). The exclusion criteria are women who used antimicrobials during the previous month before sampling.
Microbial culture
Undiluted 50 FF samples were inoculated directly using 100 µl of the sample was spread by L-loops in triplicate on top of Sabouraud Dextrose agar medium (SDA), Potato dextrose agar (PDA), 65 g of Yeast peptone dextrose agar (YPD) (Himedia, Mumbai, India) with 10% Lactic Acid to inhibits the bacterial growth [17]. All plates were incubated aerobically at 25°C and 37°C for 2-5 days because the most clinically important fungi have an optimum growth temperature at 25°C and 37°C for thermal types [18, 19]. All procedures of fungal isolates were performed under sterilized conditions inside the Biosafety cabinet (Daihan Lab tech, Kyungki-Do, South Korea).
Morphological structures analysis
All isolates were phenotypically identified from pure cultures. Colony characteristics including growth rate, colony color, texture, size, and shape were considered as important diagnostic criteria for identification and recorded as macro-morphological structures. The micro-morphological structure was observed in wet mounts prepared on microscopic slides and stained with lactophenol cotton blue (LPCB) stain using the standard protocol [20, 21].
Microbial analysis
The total number of colonies was done by manually counting of colonies on plates and the results were calculated as the mean of colony-forming units per milliliter (CFU/ml). The limit of detection was 103 CFU/ml as described by Pelzer [22].
Biochemical analysis
Different biochemical analysis tests were conducted to differentiate between the yeast colonies using the VITEK 2 system (bioMérieux, Durham, USA) by comparison of the biochemical profile with an extensive database. 46 biochemical tests are measuring multi carbon source utilization, nitrogen source utilization, and enzymatic activities. The results were obtained automatically, and the identification of the unknown organism was described according to a specific algorithm. The final identification of excellent, very good, good, acceptable, or low discrimination was correct.
For short term storage, individual colonies of isolates were inoculated on slant agar in universal glass tubes with a screw cap and stored at 4°C until used [23].
For long-term storage, all the isolates were preserved at −80°C in YPD broth ((Himedia, Mumbai, India)) containing 10% (1:1) sterile glycerol (Sigma-Aldrich, Missouri, USA) [24].
Molecular identification of microbial isolates
DNA extraction and PCR amplification
The DNA was extracted using a genomic BYF DNA extraction mini kit (Thermo Scientific, USA, catalog No. 78870) according to the manufacturer's instruction and suspended in 50 µl lysis buffer. The PCR technique was performed by amplifying the internal transcribed spacer (ITS) region of the ribosomal DNA (rDNA) using universal primers ITS1 (CTTGGTCATTTAGAGGAAGTAA) and ITS4 (TCCTCCGCTTATTGATATGC) using a thermal cycler (Veriti Thermal Cycler, Applied Biosystems, USA). The 50 μl reaction mixture contained 3 μl of template DNA, 2 μl each of the primers, 25 μl of green PCR mix (Promega, Go Taq ® Green Master Mix, USA), and 18 μl of PCR grade water. The PCR program was as follows: an initial denaturation of 2 min at 96 °C, followed by 35 cycles at 94 °C for 1 min, at 56 °C for 45 s, at 72 °C for 1 min, and final extension cycle at 72 °C for 6 min. [23]. The negative control was prepared with the reaction mixtures in the absence of DNA extract [25]. The DNA was quantified using a Nanodrop spectrophotometer (Nanodrop 2000, Thermo Scientific, Massachusetts, USA). The absorbance ratio (A260/280) of 1.8−2.2 was used for PCR analysis and stored at −20°C until required [24].
DNA Visualization
The PCR products were loaded onto 1.5 % agarose gel prepared in 1X Tris Acetate-EDTA (TAE) buffer (Thermo Scientific, Massachusetts, US) with ethidium bromide added to a final concentration of 1 µg/ml. Amplicons were run for 45 min at 130 volts using electrophoretic gel (Horizontal gel electrophoresis, Cleaver Scientific, UK). The DNA marker (100 bp, Invitrogen, USA) was used to quantify and identify PCR products. All sample bands were visualized under UV light (gel doc imager, Bio-Rad, USA) to evaluate the DNA quality. Then the samples were sent to Macrogen Company, Seoul, South Korea for purification and sequencing.
DNA Sequencing
The sequence results for each PCR product were assembled using the Big Dye terminator cycle sequencing kit (Applied Biosystems, U.K). Sequence identities were characterized using BLAST GenBank general databases from the National Center for Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov) [23]. The sequence alignment of the constructions of neighbor analysis was done by the BLAST and MEGA-X program (version 10.0.5). The result reports were received from the Microgen molecular analysis laboratory.
Antimicrobial susceptibility test
The susceptibility to five antifungal agents was determined using the VITEK 2 antifungal susceptibility testing system (biomatrix, Durham, USA) (card number AST-YS08) to determine the antifungal susceptibility of yeasts compared with the Clinical and Laboratory Standards Institute (CLSI M100-S25, 2015) reference standards within 24 hours. The antifungal used included Caspofungin (CAS) (0.25-4 ug/ml), Fluconazole (FLU) (1-64ug/ml), Flucytosine (FCT) (1-64ug/ml), Micafungin (MCF) (0.06-4 ug/ml), and Voriconazole (VRC) (012-8ug/ml). The cassettes were loaded and placed into the VITEK-2 instrument, and the suspensions were diluted by the instrument. Afterward, the cards were filled, incubated, and read automatically. The complete data for each fungal isolate were expressed as minimal inhibitory concentrations (MICs) in micrograms per milliliter (µg/ml).
Statistical Analyses
Data collected were analyzed using SPSS25.0 to determine the significant difference among the data (Armonk, NY: IBM Corp). The data were tested using a Kolmogorov–Smirnov test, Shapiro–Wilk tests, Chi-square test (χ2), T-test, and ANOVA test. A p-value of < 0.05 was considered statistically significant.
RESULTS
Patient demographics
Clinical specimens were collected from 50 women for microbiological analyses.
The mean (±SD) age of all women was (35.8 ± 5.47) years. The mean age of infertile women (37.03 ± 5.26 years) compared with (33.19 ± 5.12) for fertile women (P=0.019). Also, the mean weight of infertile women was (74.21 ± 15.14 kg) and (63.29 ± 12.64 kg) of fertile women (P=0.016). Furthermore, the mean number of oocytes retrieved for fertile women was (13 ± 7 eggs) compared with (11 ± 6 eggs) for infertile women (P=0.046) as shown in Table [1]. The positive IVF outcomes were 28% while 72% for negative IVF outcomes. The mean age of women with positive IVF outcomes was (33.71 ± 5.11 years) and negative IVF outcomes were (36.61 ± 5.31 years). However, the age of women was not associated with IVF outcomes (P=0.093). Furthermore, the IVF outcomes were not associated with the etiology of infertility (P>0.05) and ovulatory disorders were the most common causes of infertility with negative IVF outcomes 38%. However, there was no statistically significant difference in the IVF outcomes with the presence of microbes (P˃0.05).
Isolation and identification of yeast isolates
Microorganisms were detected within 5 (10%) FF samples and 45 (90%) did not show any fungal growth.
In the microscopic specimens, five isolates showed yeast cells with (LPCB) stain. The morphological features were identified up to the genus level according to the morphological reference published [21]. The features of all isolates are creamy colored to yellowish and the texture of the colony was smooth, glistening, or dry, depending on the species. Generally, the microscopic features of all isolates showed yeast species with blast conidia produce singly or in small clusters and round or elongate with a variable in size between 2.0 to 7.0 μm as shown in Figure [1, 2, and 3].
|
Table 1: The relation between patient demographics with women's fertility. |
|||
|
Patient demographics |
Groups |
P-value |
|
|
Infertile women 34 (68%) |
Fertile women** 16 (32%) |
||
|
Mean ±SD |
Mean ±SD |
||
|
Age |
37.03 ± 5.26 |
33.19 ± 5.12 |
0.019* |
|
Weight |
74.21 ± 15.14 |
63.29 ± 12.64 |
0.016* |
|
Number of oocytes |
9 ± 5 |
13 ± 7 |
0.046* |
*P-value < 0.05 is significant **Fertile women with infertile male partners.
The effect of the microbial load was also considered in this study. The quantitative method assessed the prevalence of yeast within the FF samples. The yeast species were isolated at concentrations ranging from 102 CFU/ml to >103 CFU/ml in FF samples. There appeared to be no correlations between the microbial load in FF and the etiology of infertility. The yeast colonies grew on the three different media with two different temperatures 25°C and 37 °C but with the different growth rates.
The total CFU/ml of all yeast colonies isolated from different media at 25°C were 22.03×103 CFU/ml whereas 24.48×103 CFU/ml at 37°C. In the PDA medium, the total CFU/ml of yeast colonies grown at 25°C were 7.3×103 CFU/ml while the total CFU/ml grown at 37°C were 7.73×103 CFU/ml. Furthermore, the total CFU/ml of yeast colonies grown on SAB medium at 25°C were 7.23×103 CFU/ml, and 8.21×103 CFU/ml at 37°C. In the YPD medium, the total CFU/ml of yeast colonies grown at 25°C were 7.5×103 CFU/ml while the total CFU/ml grown at 37°C were 8.54×103 CFU/ml.
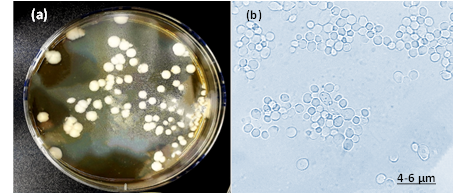
Fig. 1: The isolate of C. Albicans (a) colonies represents creamy color, irregular, convex, smooth and pasty colonies on YPD agar (b) Cellular morphology of yeast cells stained with LPCB and observed blastoconidia produce in clusters in size 4-6 μm under 100x objective by light microscopy.
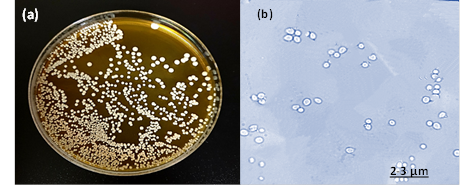
Fig. 2: The isolate of C. glabrata (a) colonies represents creamy color, circular, convex, smooth and glistening colonies on YPD agar (b) Cellular morphology of yeast cells stained with LPCB and observed small blastoconidia produce singly in size 2- 3 μm under 100x objective by light microscopy.
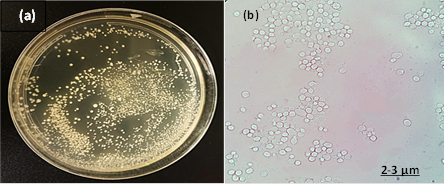
Fig. 3: The isolate of C. guilliermondii (a) colonies represents creamy-yellow color, circular, flat, smooth, and moist colonies on SAB agar (b) Cellular morphology of yeast cells stained with LPCB and observed small blastoconidia produce in clusters in size 2- 3 μm under 100x objective by light microscopy.
All yeast isolates were successfully identified at the species level by the VITEK-2 system as one isolate of C. albicans (95%), one isolate of C. guilliermondii (99%), and three isolates of C. glabrata with the probability (96–99–99 % respectively). The time to identification with VITEK 2 YST cards was 18 h.
Five yeast isolates were identified and confirmed using molecular analytical techniques. All yeast isolates belong to one genus and three species which were identified as Candida glabrata (60%), C. albicans (20%), and C. guilliermondii (20%).
The PCR amplification of DNA extracted from the yeast strains was done with two universal primers pairs. The intense sharp bands on agarose gel appeared the PCR product with a molecular size of ITS1/4 in the range from 535 to 871 for all yeast strains as shown in Figure [4].
This was followed by PCR genetic sequences of all strains that were aligned with the available sequences of closely related strains accessed from the GenBank. Molecular identification of strains to species level based on 99–100% similarity with sequences of the known species already published in NCBI databases as shown in Table [2].
The dendrogram showing phylogenetic analysis indicted the taxonomic relationship based on the ITS1/4 region of three Candida species aligned with closely related sequences accessed from the GenBank as shown in Figure [5]. All Candida species belong to the same order which is Saccharomycetales and grouped into phylum Ascomycota.
|
Table 2: List of yeast strains GenBank accession number isolated from FF and % identity with closely related strains found in the NCBI website. |
|||
|
Sample code |
Closet related species |
Gene bank no. |
Similarity |
|
F1 |
C. albicans |
CP032012.1 |
100% |
|
F2 |
C. glabrata |
MH734842.1 |
99% |
|
F3 |
C. guilliermondii |
MK394108.1 |
99% |
According to CLSI documents the Candida isolates indicate different antifungal sensitivity to five antifungal agents including CAS, FLU, FCT, MCF, and VRC except for three C. glabrata strains that not showed any MIC values against fluconazole with an analysis message (this antibiotic is not claimed). Micafungin showed the highest susceptibility to three isolates of C. glabrata at (≤ 0.06 µg/mL) and VRC showed the highest susceptibility to the other two isolates of C. albicans and C. guilliermondii at (≤ 0.12 µg/mL) as shown in Table [3].
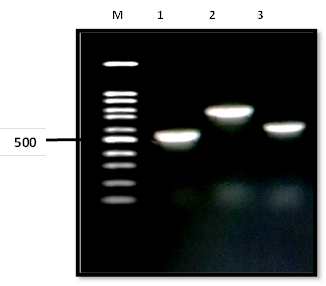
Fig. 4: Gel electrophoresis of PCR product of three Candida species with ITS1/4 regions where lane 1: represents C. albicans, lane 2: indicates C. glabrata, lane 3: indicates C. guilliermondii. The range of DNA isolates (1- 3) molecular size of bands was from 535 to 871 bp compared to the standard M (100 bp molecular size marker).
|
Table 3: Antifungal susceptibilities of Candida isolates from FF samples as determined by the VITEK 2 system. |
||||
|
Yeast species |
Antifungal agent |
MIC (µg/mL) |
||
|
Range |
Susceptible |
|||
|
C. albicans |
CAS |
0.25- 4 |
0.5 |
|
|
FLU |
1- 64 |
2 |
||
|
FCT |
1- 64 |
≤ 1 |
||
|
MCF |
0.06- 4 |
0.5 |
||
|
VRC |
0.12- 8 |
≤ 0.12 |
||
|
C. glabrata |
CAS |
0.25- 4 |
0.12 |
|
|
FLU |
1- 64 |
NS |
||
|
FCT |
1- 64 |
≤ 1 |
||
|
MCF |
0.06- 4 |
≤ 0.06 |
||
|
VRC |
0.12- 8 |
≤ 0.12 |
||
|
C. guilliermondii |
CAS |
0.25- 4 |
0.25 |
|
|
FLU |
1- 64 |
2 |
||
|
FCT |
1- 64 |
≤ 1 |
||
|
MCF |
0.06- 4 |
0.5 |
||
|
VRC |
0.12- 8 |
≤ 0.12 |
||
Scale azole of the minimum inhibitory concentration (MICs) was measured using the VITEK-2 system. (S) Susceptible; (R) Resistant. CAS, caspofungin; FLU, fluconazole; FCT, flucytosine; MCF, micafungin; VRC, voriconazole; NS, MIC value not showed with analysis message (this antibiotic is not claimed).
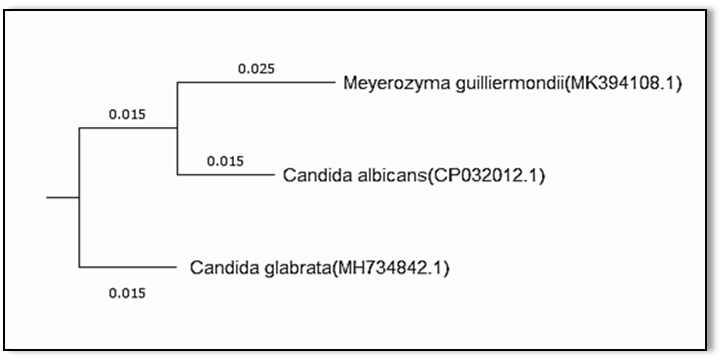
Fig. 5: Dendrogram showing phylogenetic analysis based on the ITS4/1 region and NCBI GenBank database for three Candida species.
DISCUSSION
This study investigated the presence of yeast within fifty FF specimens collected from women undergoing IVF procedure with different causes of infertility and the effect of these species isolated on the IVF outcomes. The mean age and weight of women were statistically significantly higher in the infertile women group. This data is in agreement with what has been published by others when women reach 35 years of age, their fertility is declining [7, 26, 27]. Several studies have shown an increased risk of infertility in obese women [7, 28, 29]. Also, the mean number of oocytes was the highest in infertile women with infertile male partners (male factor). This result is incompatible with the finding of Pelzer, Allan [13] which reported that there was no difference in the number of oocytes collected at the time of retrieval for fertile and infertile women. On the other hand, the age of women was not associated with IVF outcomes. This result is inconsistent with the findings of [30, 31] which concluded that IVF outcome is strongly correlated with maternal ages. Furthermore, we have shown that the presence of yeast within FF does not correlate with the etiology of infertility.
In the present study, one genus including three species was isolated from five FF specimens from different women. The Candida isolates were present in 10% of FF tested while there were 90% of FF did not show any fungal growth. Overall 50 cases, we stated that ovulatory disorders and malefactors were the most common causes of infertility (44%, and 32%, respectively). We concluded that the presence of yeasts [Candida albicans, Meyerozyma guilliermondii (anamorph Candida guilliermondii), and Candida glabrata] were not correlated with adverse IVF outcomes. In agreement with our findings, previous studies have also reported that C. albicans was one of the microorganisms detected in the FF and have not been associated with either the etiology of infertility or the IVF outcomes [13]. In one retrospective study, the IVF cultures for 5/729 patients contaminated by yeast species and the embryo quality were not compromised and all five patients conceived [10]. This evidence further not supports our hypothesis that FF microorganisms may influence the success rate of the IVF procedure.
The FF may colonize by the hematogenous invasion of different microbes spread via the oral mucosa and respiratory tract. It is may also be contaminated by vaginal microbes when the embryologist starts to collect the sample by a large needle passed through the ovary. In our study, Candida spp. is a common commensally yeast of the female genital tract and considered as opportunistic pathogens that may occasionally cause diseases and it can be transmitted from the colonized mother’s vagina to the newborn, leading to congenital candidiasis infection [32, 33]. Candida albicans infection occurs mostly (80% to 90%) of diagnosed cases, while infection with other species occurs less frequently, such as C. glabrata and C. tropicalis [34]. Candida albicans is one of the microorganisms isolated from cases of pelvic inflammatory diseases [35]. Selman, Mariani [36] also reported the effect of fungal contamination on IVF outcomes isolated from the vagina, cervix, embryo culture medium, and tip of the catheter. They found that the appearance of yeasts in 28 patients (18.3%) did not demonstrate any effect on pregnancy outcomes. Mahmoudi Rad, Zafarghandi [37] studied the vaginal swab specimens from women with VVC and they reported that C. albicans and C. glabrata were prevalent species isolated from them and the most common causes of VVC. Over 10% of all the candidemia cases are caused by M. guilliermondii in some regions such as Brazil, India, and Italy [24].
The yeast isolated from FF samples recovered in this study at concentrations ranging from 102 CFU/ml to >103 CFU/ml and there were no correlations between the microbial load and the etiology of infertility. Another study showed that most microbial species were isolated at concentrations ranging from 103 CFU/mL to >106 CFU/mL in both colonized and contaminated FF and the microbial load was not associated with the etiology of infertility or the IVF outcomes [22].
The quantitative method assessed the prevalence of yeast within the FF samples isolated on the three media (PDA, SAB, and YPD) with two different temperatures 25°C and 37 °C. We found that the yeast colonies grew on the three media but with the different growth rates. We concluded that the yeast colonies were formed rapidly on the YPD medium at 37°C. This result is consistent with the finding of Odds and Davidson [38] which concluded that the growth of yeast colonies tended to be greater and faster at 37 °C on the CHROM agar medium.
The yeast isolates identified using the VITEK 2 automated microbiology system by measured 46 biochemical tests including carbon source utilization, nitrogen source utilization, and enzymatic activities. Melhem, Bertoletti [39] identified 32 clinical isolates of yeast by the VITEK 2 system and they showed that all strains 100% correctly identified. Hata, Hall [40] studied 623 clinical yeast isolates and they discovered an error in identification using the VITEK 2 system for one clinical isolate of C. glabrata. However, we did not observe any misidentification results in our study.
To ensure suitable differentiation and confirmation of the isolates at the species level, we used the advanced molecular technique based on amplifying the ITS1 segment for the yeast isolates using PCR products and sequencing analysis method. Several researchers reported fungal species based on this technique to confirm species identification [23]. The molecular identification for the microbial strains revealed that the identification of microbial isolates using the morphological features and the biochemical tests were correct, thus yielding the correct identification for all isolates tested. The molecular size of the ITS region of C. albicans was approximately 535 bp, and 871 bp of C. glabrata similar to that reported by [41]. C. guilliermondii showed a band size of 607 bp, in agreement to that observed by [24].
Furthermore, the embryo culture medium does not routinely contain any antifungal agents, which is the cause of the common fungal contamination in IVF culture [10]. Also, resistance to antifungal agents has considered an important challenge in public health problems, especially if the patient has been in the past treated with an azole group [32]. Also, the decrease of susceptibility and the emergence of strains resistant to antifungal agents like polyene (amphotericin B) and azoles (fluconazole and itraconazole) for C. guilliermondii and C. glabrata is always increasing the threat by this organism [24, 42]. In our study, we have studied the effects of five antifungal agents on the yeast isolates using the VITEK 2 automated microbiology system and we reported that MCF and VRC showed the highest susceptibility against all Candida species isolated. Regarding the other studies, Khan, Ahmed [32] reported that FLU was extremely resistant against Candida spp. followed by clotrimazole and nystatin. On the other side, VRC had the highest antimicrobial activity against Candida spp. Małek, Paluchowska [43] evaluated the susceptibility of Candida isolates including (C. albicans, C. glabrata, C. tropicalis, C. krusei, and C. kefyr) to several antifungal agents and they reported that all Candida spp. were susceptible to many antifungal agents including (amphotericin B, FLU, 5-fluorocytosine, and VRC) except for two C. glabrata strains that showed dose dependent susceptibility to FLU.
CONCLUSION
This study investigated the fungi within FF of women undergoing IVF cycles. Follicular fluid is not always sterile, but it contains different species of yeasts that are not correlated with the IVF outcomes and a larger sample is needed to further examine our hypothesis. Also, microbiological examination of FF in women who have undergone failed IVF cycles may provide an opportunity to initiate antimicrobial therapy before the next conception.
ACKNOWLEDGMENT
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah supported this project. The authors have acknowledged this and with thanks DSR for technical and financial support.
Ethical approval:
Ethics approval was obtained from the Biomedical Ethics Unit at King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
Conflict of Interest
None.
REFERENCES
- Kumar, N. and A.K. Singh, Trends of male factor infertility, an important cause of infertility: A review of literature. Journal of human reproductive sciences, 2015. 8(4): 19
- World health organization, W. Multiple definitions of infertility. 2016.
- Mascarenhas, M.N., et al., National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS medicine, 2012. 9(12): e1001356.
- Al-Turki, H.A., Prevalence of primary and secondary infertility from tertiary center in eastern Saudi Arabia. Middle East Fertility Society Journal, 2015. 20(4): 237-240.
- World Bank, W.b., Fertility rate, total (births per woman). 2017, United Nations Population Division.
- Anwar S, Anwar A. Infertility: A review on causes, treatment and management. Womens Health Gynecol. 2016;5:2-5.
- Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Human reproduction update. 2007 May 1;13(3):209-23.
- Aoun TB, Moawed S. Effects of environmental, cultural, and socioeconomic factors on Saudi infertile couple in Riyadh City. Life Science Journal. 2012;9(4):4861-
- Pelzer ES, Allan JA, Cunningham K, Mengersen K, Allan JM, Launchbury T, Beagley K, Knox CL. Microbial colonization of follicular fluid: alterations in cytokine expression and adverse assisted reproduction technology outcomes. Human reproduction. 2011 Jul 1;26(7):1799-812.
- Pomeroy, K., Contamination of human IVF cultures by microorganisms: a review. J Clin Embryo, 20 13: 11-30.
- Basuino, L., Silveira, J. C. Human follicular fluid, and effects on reproduction. JBRA assisted reproduction, 2016. 20(1): 38-40.
- Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reproductive biology and endocrinology. 2009 Dec 1;7(1):40.
- Pelzer ES, Allan JA, Waterhouse MA, Ross T, Beagley KW, Knox CL. Microorganisms within human follicular fluid: effects on IVF. PloS one. 2013 Mar 12;8(3):e59062.
- Hamad, T.A., et al., Microbial colonization of human follicular fluid and adverse outcome on in vitro fertilization cases in Kamal al-Samarrai's Hospital for fertility and In vitro fertilization treatment in Baghdad, Iraq. Dental and Medical Sciences 2018. 17(5): p. 80-87.
- Ibadin KO, Osemwenkha AP. Microbiological study of infertile women programmed for invitro fertilization–embryo transfer in a tertiary health institution In Nigeria.
- Kim S, Won K, Lee J, Suh C. The incidence of positive bacterial colonization in human follicular fluids and its impact on clinical in vitro fertilization outcomes. Fertility and Sterility. 2018 Sep 1;110(4):e194.
- Madhavan P, Jamal F, Chong PP. Laboratory isolation and identification of Candida species. JApSc. 2011 Dec;11(16):2870-7.
- Lion, T., Human Fungal Pathogen Identification: Methods and Protocols. 2017: Springer.
- Mulla AF, Shah AA, Koshy AV, Mayank M. Laboratory diagnosis of fungal infection. Universal Research Journal of Dentistry. 2015;5:49-53.
- Carter GR, Cole Jr JR, editors. Diagnostic procedure in veterinary bacteriology and mycology. Academic Press; 2012 Dec 2.
- Kidd S, Halliday CL, Alexiou H, Ellis D. Descriptions of medical fungi. the Authors; 2016.
- Pelzer, E.S., Microbial colonization of human follicular fluid and adverse in vitro fertilization outcomes. 2011, Queensland University of Technology.
- Najjar, A., et al., Seasonal Variations of Fungi Isolated from Swimming Pools in Jeddah, Saudi Arabia. Middle-East Journal of Scientific Research 2019. 27(1): p. 55-63.
- Romi W, Keisam S, Ahmed G, Jeyaram K. Reliable differentiation of Meyerozyma guilliermondii from Meyerozyma caribbica by internal transcribed spacer restriction fingerprinting. BMC microbiology. 2014 Dec 1;14(1):52.
- Alnefaie, Z., Mutawakil, M.H., Ahmed, M.M.M. Molecular Identification for Camel Udder Microbiota. Advances in Environmental Biology, 2019. 13(4): 7-13.
- Baird, D., et al., Fertility, and aging. Hum Reprod Update 2005. 11: 261-276.
- Pal, L., Santoro, N. Age-related decline infertility. Endocrinology and metabolism clinics of North America, 2003. 32(3): 669-688.
- Rich-Edwards JW, Spiegelman D, Garland M, Hertzmark E, Hunter DJ, Colditz GA, Willett WC, Wand H, Manson JE. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002 Mar 1;13(2):184-90.
- Metwally, M., T.C. Li, W.L. Ledger, The impact of obesity on female reproductive function. Obesity Reviews, 2007. 8(6): 515-523.
- Nahum R, Shifren JL, Chang Y, Leykin L, Isaacson K, Toth TL. CLINICAL ASSISTED REPRODUCTION: Antral Follicle Assessment as a Tool for Predicting Outcome in IVF—Is it a Better Predictor than Age and FSH?. Journal of assisted reproduction and genetics. 2001 Mar 1;18(3):151-5.
- Yan J, Wu K, Tang R, Ding L, Chen ZJ. Effect of maternal age on the outcomes of in vitro fertilization and embryo transfer (IVF-ET). Science China Life Sciences. 2012 Aug 1;55(8):694-8.
- Khan M, Ahmed J, Gul A, Ikram A, Lalani FK. Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infection and drug resistance. 2018;11:447.
- Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, Ambrosini G, Armanini D. Genital tract infections and infertility. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2008 Sep 1;140(1):3-11.
- Soong D, Einarson A. Vaginal yeast infections during pregnancy. Canadian family physician. 2009 Mar;55(3):255.
- Okiki PA, Amiegheme NE, Oyinloye J. Evaluation of microorganisms associated with vaginal infections in Owo, Nigeria. ARX and ARMAX Modelling of olefin metathesis reactive distillation process. 2015;6(9):79-83.
- Selman H, Mariani M, Barnocchi N, Mencacci A, Bistoni F, Arena S, Pizzasegale S, Brusco GF, Angelini A. Examination of bacterial contamination at the time of embryo transfer, and its impact on the IVF/pregnancy outcome. Journal of assisted reproduction and genetics. 2007 Sep 1;24(9):395-9.
- Mahmoudi Rad M, Zafarghandi AS, Amel Zabihi M, Tavallaee M, Mirdamadi Y. Identification of Candida species associated with vulvovaginal candidiasis by multiplex PCR. Infectious diseases in obstetrics and gynecology. 2012 Oct;2012.
- Odds FC, Davidson A. “Room temperature” use of CHROMagar Candida™. Diagnostic microbiology and infectious disease. 2000 Nov 1;38(3):147-50.
- Melhem MS, Bertoletti A, Lucca HR, Silva RB, Meneghin FA, Szeszs MW. Use of the VITEK 2 system to identify and test the antifungal susceptibility of clinically relevant yeast species. Brazilian Journal of Microbiology. 2013 Dec;44(4):1257-66.
- Hata DJ, Hall L, Fothergill AW, Larone DH, Wengenack NL. Multicenter evaluation of the new VITEK 2 advanced colorimetric yeast identification card. Journal of clinical microbiology. 2007 Apr 1;45(4):1087-92.
- Vijayakumar R, Giri S, Kindo AJ. Molecular species identification of Candida from blood samples of intensive care unit patients by polymerase chain reaction–restricted fragment length polymorphism. Journal of laboratory physicians. 2012 Jan;4(1):1.
- Rodriguez-Tudela, J., et al., EUCAST Technical Note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect, 2008. 14(10): 982-4.
- Małek M, Paluchowska P, Bogusz B, Budak A. Molecular characterization of Candida isolates from intensive care unit patients, Krakow, Poland. Revista iberoamericana de micologia. 2017 Jan 1;34(1):10-6.
