Archive \ Volume.11 2020 Issue 3
Study on the Effect of Ethanol Ginger Extract on Cell Viability And p53 Level in Breast and Pancreatic Cancer
Soroush Sarami1, Maryam Dadmanesh2, Zuhair M. Hassan3, Khodayar Ghorban1*
1Department of Immunology, School of Medicine, Aja University of Medical Sciences, Tehran, Iran. 2 Department of Infectious Diseases, School of Medicine, Aja University of Medical Sciences, Tehran, Iran. 3 Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
Abstract
Cancer is a widespread disease in which regulatory mechanisms of cell growth and proliferation has led into failure and results in continuous cell reproduction. Today, breast and pancreatic cancers are two common and lethal diseases. In recent years anti-cancer effects of natural products like Ginger and its bioactive compounds has taken into consideration. In this study effect of ginger treatment was evaluated on viability and the p53 level of Panc-1 and MCF-7 Cell line. At first Cancerous and normal cells were cultured and then treated with different concentrations of ethanol ginger extract for 24 and 48 hours and eventually MTT assay was conducted to investigate the cytotoxic effect. Then we tested the p53 level in all three cells. In MTT assay we observed that ginger extract kills cancerous cell line in a dose-dependent pattern with IC50 of 40.8 and 61.5 µg/ml for MCF-7 and Panc-1 respectively, while no significant cytotoxic effect was observed on normal cells. P53 level was significantly increased in MCF-7 and Panc-1 and not in PBMCs (P<0/05). A large body of evidence demonstrated that apoptosis is a major active process in cells. Multiple mechanisms are involved in cell growth arrest and apoptosis.as reported P53 plays a key role in ginger-extract-induced apoptosis. Here our study showed ginger induces apoptosis by regulation of p53 in cells bearing mutant and wild-type p53.
Keywords: ginger, breast cancer, pancreatic cancer, p53, apoptosis, MTT
INTRODUCTION
Cancer is a multifactorial disease in which reasons such as activation of oncogenes , exposure to carcinogens and malfunction of tumor suppressor genes (like TP53) are involved [1]. In a steady-state, cell growth must be strictly balanced by an important active process of programmed cell death, called apoptosis. Failure in process of apoptosis results in uncontrolled cell replication and the inability of body to remove damaged cells which may lead to a neoplasm [2].
Increasing life expectancy, aging and unhealthy lifestyle are considered as major risk factors for cancers in both developed and developing countries. Studies have shown an ongoing increasing trend in the global incidence of cancer [3], especially in developing countries so that approximately 70% of all death cancers occur in low- and middle-income countries like Iran [4].
Cancer is now a major health problem and the second cause of death globally. It is estimated that by 2030, there would be an approximate of 26.4 million new cancer cases and 17 million people will lose their lives in the world .Among all kinds, Breast cancer due to high incident, and pancreatic cancer, because of relatively low survival rate, are the two noteworthy ones [3].
Today breast cancer is considered as the most prevalent cancer among females the third most diagnosed cancer worldwide [5]. Because of metastasis, which is when cancerous cells alter their microenvironment, enter the circulation, and colonize at distant organs, and despite significant advances toward targeted therapy and screening techniques, breast cancer is considered as the second leading cause of cancer-related mortality among females specially in developing countries [6, 7]. Despite the fact that many tumors respond to treatment at first, breast cancer cells may survive and acquire resistance to common drugs [8].
Gastrointestinal cancer is defined as malignancies in the gastrointestinal tract organs such as stomach, esophagus, small intestine, biliary system, rectum, large intestine, anus and as well pancreas [9].
In the United States, %20 of all newly diagnosed cancer cases are GI cancers. Among different kinds of GI cancers, pancreatic carcinoma has one of the poorest prognosis and its incidence is gradually increasing during the past several decades. Pancreatic Cancer is an aging condition and its occurrence is 50% higher in men than women [10, 11].
Pancreatic cancer is an aggressive neoplasm that develops in a relatively symptom-free manner with many drug-resistant types, so that most of the cases has already become metastatic at first diagnosis, which shows the importance of prevention in this disease. The 5-year survival rate of pancreatic cancer is less than 10% and it remains as one of the five most common causes of cancer death in developed countries and by 2030, it might be the second cause of deaths (cancer-related deaths) in the United States [12, 13]. As Resistance of recurrent disease to common drugs is the major problem in limiting long-term treatment success, further and more comprehensive studies and novel drugs are needed in this field. Nowadays dietary intervention is well-established and accepted as a way of suppressing or inhibiting cancer growth before it develops into advanced stages. While comprehensive studies are still needed but there is no doubt that healthy dietary compounds can delay or avoid altogether the advent of cancer specially without common side effects of usual cancer treatments [14, 15].
Laboratory and epidemiological studies has provided indubitable proof, suggesting that increased and regular intake of fruits, vegetables and spices, alongside with a healthy lifestyle, are strongly linked with remarkable anticancer effects [16]. There is this thought that regular dietary prevalence of foods such as ginger, turmeric , garlic , broccoli , green tea and grape seed extracts in South East Asian countries contribute to the decreased incidence of several types of cancers [17].
indisputable evidence show that among all natural products, ginger (Zingiber officinale), which is extensively used in foods and beverages as a spice worldwide, is a unique source of bioactive compounds that can show both chemo-preventive and chemotherapeutic effect against different types of cancers and has attracted the interest of medical scientists recently [18].
Ginger is a rhizomatous plant with more than 400 different compounds and has been widely used as a herbal treatment for a wide range of disorders such as dyspepsia, colic, nausea, vomiting, gastritis, flatulence, loss of appetite, diarrhea [19], migraine headaches [20], atherosclerosis and hypertension [21] and common cold [22] for years.
Recently, accumulating evidence suggests that whole ginger extract In addition to its numerous bioactive components like gingerols, shogaols, paradols, and zingerone may exhibit many biological effects including immuno-modulatory, Antioxidants, anti-inflammation and anti-carcinogenic properties [23, 24].
In addition, common current anti-cancer therapies, despite their great benefits, cannot distinguish between cancerous and healthy cells, which may lead to poor therapeutic effect and significant side effects. Therefore, new treatments with selective toxicity are needed.
Considering previously reports and based on the fact that in some studies herbal extracts are more effective than the a single purified components [25], this study was aimed at examining the cytotoxic and apoptotic effect of crude ginger extracts on two cancer cell lines.
One of the numerous cellular pathways that ginger performs its anticancer properties is inducing cell cycle arrest and apoptosis in cancerous or transformed cells through p53signal pathway [26].
p53 gene was first described in 1979 and believed to be an oncogene, but later it was reported as a tumor suppressor gene. P53 is involved in some cellular pathways such as apoptosis, DNA repair, senescence, metastasis, invasion, stem cell maintenance and cell cycle arrest [27]. In normal cellular environment the tumor suppressor p53 is in its standby mode, but in response to different cellular stresses such as DNA damage and expression of oncogenes, it gets activated and hinders abnormal cell proliferation, induces apoptosis and therefore prevents neoplastic development. Defective or mutated p53 could allow transformed cells to proliferate. So that approximately 50% of all human tumors contain p53 mutants [28] since the tumors that consist of mutant p53-expressing cells show resistance to common anti-cancer drugs. Manipulating and disrupting this fatal resistance is a considered as the main challenge in cancer therapy [27].
With this mindset, the present study aimed to examine the cytotoxic and anti-proliferative potentiality of whole ginger extract on two well-characterized cell lines: MCF-7, as a cancerous cell with wild type p53, and Panc-1 as a cell with mutated p53. Then, the selectivity of the effect of ginger extract toward cancer cells was addressed. For this purpose, we used PBMCs (peripheral blood mononuclear cell) as normal cell line.
MATERIAL AND METHOD:
- Cell culture
Human breast cancer cell line, MCF-7 and Human pancreas carcinoma, PANC-1, were both obtained from cell bank of Iranian Biological Resource Center, Tehran, Iran. (IBRC code C10082 and C10156 respectively). Briefly, Cells were cultured in RPMI 1640 medium (Gibco, UK) containing 2mM L-glutamine, 10% heat-Inactivated FBS (Gibco, UK), 100 units/ml penicillin, and 0.1 mg/ml streptomycin (Gibco, USA), as monolayer in 25 and 75 cm2 tissue culture flask (Jet Biofil) and maintained at 37 ℃ in a humidified atmosphere of 5% CO2. Medium was changed every two days and cells were subcultured at 3-4 day interval. Cells were washed with warmed phosphate-buffered saline (PBS) (DENAzist) followed by a brief incubation with trypsin/EDTA. The washed cells were isolated by centrifugation and resuspended in culture medium for plating. All experiments were performed with cells in the logarithmic growth phase and reached 70% confluence density.
- Isolation of PBMCs
PBMCs were obtained from human whole fresh blood using standard Ficoll density gradient centrifugation method. Shortly, adequate amount of human EDTA blood was diluted with equal amount of cold PBS. Then 35ml of diluted blood was carefully added over 15 ml of Ficoll (Baharafshan, Iran) in a 50ml conical tube. The tube was centrifuged in 400 g at 22℃ in a swinging-bucket rotor without brake for 20 min. The layer between plasma and Ficoll was collected With great care and washed with 5 mL PBS and centrifuged in 350g at 4℃ for 10 min, twice. PBMCs and normal cells were treated with the same concentrations of ginger.
- Preparation of ginger extract
Dry powder of the root parts of ginger rhizome, was extracted with ethanol 96% (10:1; volume for weight) for 4 hours on a magnetic laboratory shaker. The alcohol was evaporated at 65°C to get a pure ethanol extract of the ginger. The extract was weighed and dissolved in dimethylsulfoxide (DMSO) (Merck Company, Darmstadt, Germany) at the desired concentrations. The maximal dilution of DMSO in cell culture did not exceed 0.1%, even at the highest concentration of ginger. Extract was freshly prepared for each test and all studies were conducted using a single batch of ginger root extract.
- Cell growth and viability assay
The cytotoxic effect of whole ginger extract was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Merck, Germany) ( MTT) assay. To perform this test 1 × 104 cells (MCF-7 and Panc-1) were seeded into each well of a 96 well-plate in 200 μl of RPMI 1640 medium, after a 24 hour incubation and allowing cells to attach, the culture medium was removed carefully and replaced with fresh medium containing 0.0, 12.5, 25, 50, 100 and 200 µg/mL of ginger and incubated for 24 and 48 hours at 37°C in a 5% CO2 environment. Equal volumes of solvent alone (DMSO) was added to negative control cells too. After 24 and 48 hours the medium was removed and 200 μl of fresh medium and 20 μl of MTT solution (5 mg/ml in PBS) was added to each well. After 4 h of incubation in 37 ℃, the blue formazan crystals were dissolved in 100 μl of DMSO. Following 15 minutes of agitating the plates, the optical density of the solubilized formazan product in each well was measured using a micro plate reader (LabSystems Multiskan) at 570 nm with background subtraction at 650 nm. Three independent experiments were done and results were expressed as means ± SEM. The same process was performed on human PBMCs at a density of 1×105 cells per well. The viability of cells was as follows which considers the percentage of control: Viability (%): OD samples+ (OD Control – ODDMSO) /ODcontrol×100
- Determination of p53 level
We used the human p53 antibody ELISA (Enzyme-linked immunoabsorbant assay) kit (Bioassay Technology Laboratory) aimed at determining the ginger extract effect on the level of p53. Panc-1, MCF-7 and PBMCs (2 × 106 cells/well) were plated in 24-well microtiter plates and allowed to attach for 24 h at 37°C and 5% CO2. Then ginger extract was added to cell culture medium at desired concentrations (0 to 40 µg/ml ) .After 12 and 24 h, cells were trypsinized and their viability was found using trypan blue dye exclusion assay and desired number of cells (according to the kit instruction) were frozen in -80°C for further investigations. ELISA assay was performed according to manufacturer’s instruction and finally the optical density (OD value) of each well was immediately determined at 420 nm in an ELISA microplate reader (Stat Fax 2100). The experiment was repeated independently to confirm the results.
- Statistical analyses
All data were analyzed using GraphPad software version 6. At first, normality of the data and homogeneity of variances were tested with Shapiro–Wilk test. P values less than 0.05 were considered as statistically significant. The data are presented here as mean ± SD of independent experiments.
RESULTS:
Evaluation the cytotoxicity effect of ginger in vitro:
To examine the cytotoxic effects of the extract on MCF-7, Panc-1 and PBMCs the MTT assay was performed. The results in figure 1 and 2 showed that the viability of cancer cells is significantly reduced after 24 and 48 hours of treatment with ginger. And the difference is also significant between MCF-7 and Panc-1 cells. No significant cytotoxic effect was noticed on the normal cells and the viability is obviously higher in these cells. The microphotographs depicted in Figure 3 shows that the treatment of MCF-7 and Panc-1 cells with ginger extract for 24 h resulted in so apparent cell shrinkage, detachment of cells, and loss of the originally confluent monolayer.
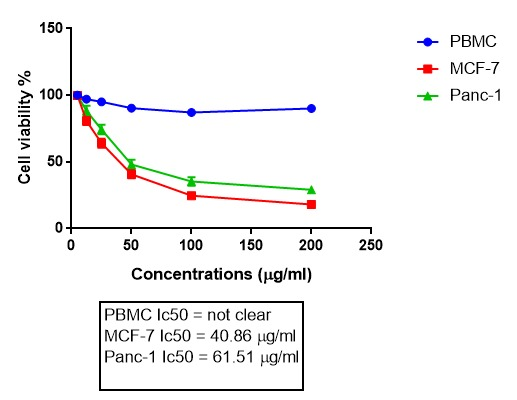
Figure 1. Cytotoxic effects of ginger on three cell lines in 24 h. The experiment was repeated three times, and the cell viability of the cells is expressed by percent of control (DMSO alone) at each dose of ginger and the result is reported as the mean±SD. P<0.05. IC50. Statistical analysis of the data was done using two way ANOVA.
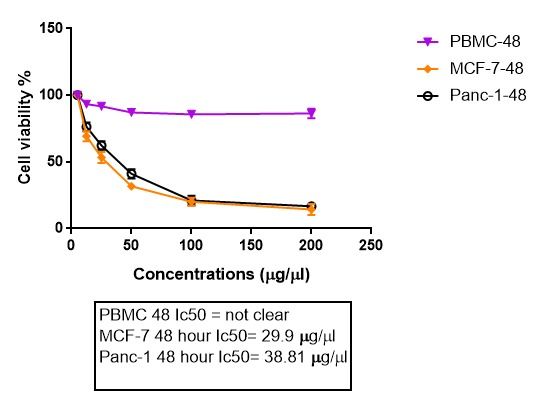
Figure 2. Cytotoxic effects of ginger on three cell lines in 48 h.
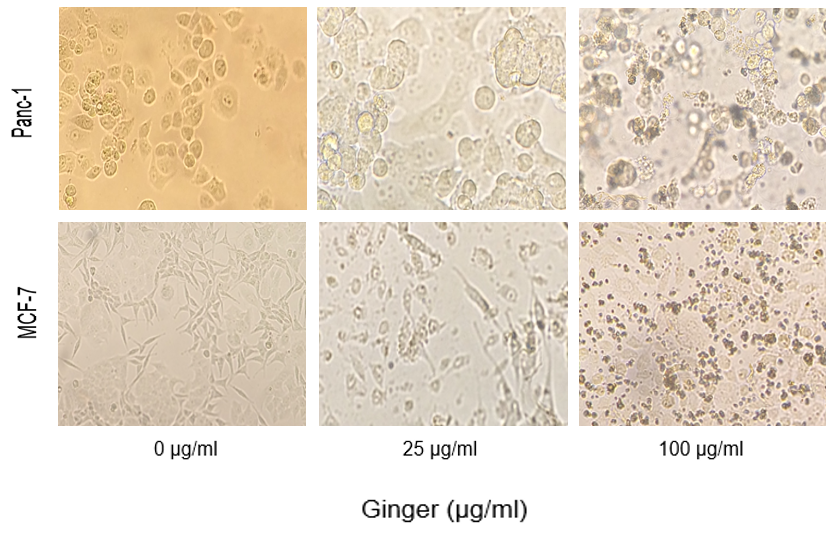
Figure 3. Microscopic photo showing the inhibitory effect of ginger extract on cellular growth of MCF-7 and Panc-1 cells in three concentrations. Cells were plated onto 24-well plates and treated with indicated concentrations of ginger for 48 h. cell shrinkage, irregularity, and detachment in ginger-treated cultures must be noticed.
Effects of ethanol ginger extract on the p53 level.
After evaluating the results of MTT assay three concentrations were chosen for treatment and then the p53 level was measured using ELISA technique in 12 and 24 hours. As shown in figure 4, p53 level increases with concentration and time. P53 basal level was determined in cells treated with similar solvent concentrations.
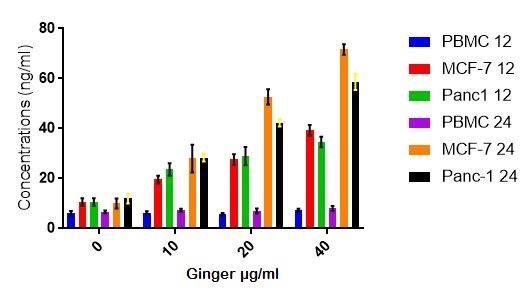
Figure 4: The level of p53 by ELISA technique in the cells treated with ginger extract. Statistical analysis of the data was performed using Non-Linear Regression.
DISCUSSION:
For many years, surgery, chemotherapy and radiotherapy have been the usual treatment strategies for breast and pancreatic cancers .Although many of these therapies have shown remarkable benefits, but still alternative treatment approaches are highly needed because the incidence of disease relapse from residual malignant cells is still an important problem. In addition, An Ideal anticancer drug is the one that completely eliminates all tumor cells with minimal damage of normal cells and does not have usual side effects of surgery, chemotherapy and radiotherapy [29]. Lately there is also an increasing interest in the develop of cost-effective, well tolerated and non-toxic natural products as an alternative medicine for the treatment of various disease like cancer.
Cancer related morbidity and mortality is a major health care concern with an ongoing increasing trend in the world. This complicated disease can be simply described as too little apoptosis and too much proliferation. Therefore induction of apoptosis and inhibition of proliferation of cancer cells has been has been introduced as a good therapeutic approach [30]. The hallmark of cancers is evading apoptosis; and activation of p53 signaling pathway is a way to defeat this evade, so one essential strategy for cancer therapy is to activate apoptotic pathways in the tumor cells [31].
On the other hand, In recent years the role of many dietary factors (alone or in combination with traditional chemotherapeutic agents) in the treatment or prevention of cancer has drawn increasing attentions. it has been made clear that the possible advantage of many naturally occurring dietary components are because of their selective anticancer activity combined with low toxicity against normal cells and few side effects [14, 32].
Among all, ginger have been known for its substantial medicinal properties for thousands of years. And its role as effective inhibitors of the carcinogenic process has recently been taken into consideration. many in vitro, animal, and epidemiological studies beside several clinical trials suggest that ginger and its active constituent suppress the growth and induce apoptosis in a wide variety of cancer types such as leukemia [33], gastric [34], prostate [35], ovarian [36], lung [37], Skin [38], and so on. However as reported here and in many other studies, normal cells are relatively resistant to ginger extract killing.
Despite the information about the potent cytotoxic activity of the ginger against cancerous or transformed cells, the molecular mechanisms responsible for this activity are not currently well clarified specially in breast and pancreatic cancers. The purpose of this research was to build an understanding of how ethanol ginger extract affects two cancer cells and determine its therapeutic value in preventing or even treating cancer.
As cells having mutated p53 lose the potent capability to elicit the enzymatic DNA repair (which is necessary to inhibit cell proliferation and to induce apoptosis) uncontrolled proliferation and malignancy may happen. So inducing apoptosis and hindering the uncontrolled proliferation is very important in such cells. Here we showed that ginger has cytotoxic and apoptotic effect on both cells bearing mutant and wild p53 [39].
The findings herein are consistent too with a report showed zerumbone (an active ginger component) reduced cellular viability of PANC-1 cells and exerted its anticancer effects by increase in ROS production and up-regulation of p53 expression. These result suggest that zerumbone, like crude ginger extract, reduces the proliferation of PANC-1 in a concentration- and time-dependent manner. miR-34 level was also increased which shows that p53 signal pathway is involved in the apoptosis process [40].
Our data shows that despite the fact that ginger has cytotoxic effects on both wild-type and mutated p53 cells, but there is a significant difference between MCF-7 and Panc-1 cells. So that IC50 was lower and p53 concentration was significantly higher in MCF-7 after 24h of treatment. This shows however P53 signaling pathway is not the only way ginger induces its anti-apoptotic affect but it is an important one. Ginger showed IC50 values at 33.78 µg/mL in HeLa cells [41] and 97 µg/ml in SKOV-3 cells [36] in In previous similar studies.
In a similar study, [6]-gingerol was capable of inducing cell cycle arrest at G1 phase and could circumvent drug resistance induced by p53 mutations [42]. Other in vivo studies show ginger extract increases the expression of p53 in Female Swiss albino mice [43].
Similar to ginger, some already reported chemotherapeutic agents with natural origin (like Cimicifuga racemose and Antrodia camphorate) also exert their anti-cancer activities by induction of apoptosis in cancer cells [44]. Another study showed that 6-gingerol suppresses metastasis and invasion of pancreatic cancer cells by preventing the disassembly of cell junctions [40]. oral consumption of whole ginger extract down regulates NFkappaB and TNF-alpha in the liver of theethionine-induced hepatoma rats [45], and inhibits prostate tumor development in both in vitro and in vivo models [35].
Clinical trials on patients with cancer receiving chemotherapy also showed that ginger reduces acute chemotherapy-induced nausea and the use of antiemetic medications too [46].
A human trial also revealed that intake of 2.0 g of ginger supplementation daily for 28 days can reduce the risk of colorectal cancers by inducing apoptosis and decreasing Bax expression [47].
in coordination with our results, a study on zerumbone-treated PANC-1 cells showed that induction of cell cycle arrest and apoptosis is associated with upregulation of p53 and p21 proteins [40]. Zerumbone also inhibits invasion of pancreatic and cancer tumor cells by regulating chemokine receptor CXCR4 expression [48].
As we know In many cell types, p53-mediated growth inhibition is dependent on induction of p21. Studies at molecular level showed that the apoptotic cell death mediated by ginger in breast cancer cells (MCF-7 and MDA-MB-231) is accompanied by decreased expression of the prosurvival genes like NFB and Bcl-X, while In contrast, p21 expression was increased, The study showed that extract did not significantly affect viability of non-tumorigenic normal mammary epithelial cell line (MCF-10A) (2) , as a result it reveals that the human breast cancerous cells are remarkably more sensitive to apoptosis induction by ginger compared with a non- cancerous mammary epithelial cell line [49, 50]. the results are compatible with our study that shows ginger increases p53 level and induces apoptosis in cells with wild-type and mutant p53 but not in PBMCs. Ginger also inhibits the growth of several tumorigenic strains of Helicobacter pylori in vitro, therefore it may have effects on chemoprevention of the gastric-intestinal cancers [51].
There are also reports that shows p53 expression was decreased by [6]-gingerol, suggesting that the induction of apoptosis is p53-independent. [6]- The resistance of mutant p53-expressing cells towards chemotherapy can be circumvented by gingerol through inducing apoptotic cell death. Unlike our study [6]-gingerol did not have cytotoxic effect in wild type p53 expressing HPAC cells [52].
Previously reports indicate apoptosis induced by ginger was not necessarily dependent on p53 wild-type status since the p53 signaling pathway was also activated in mutated p53 cells, and the extract induces apoptotic cell death in p53-mutant cancer cells as we have seen here. Thus, ginger is capable of killing and potentially eradicating mutant p53 expressing cancer cells which are or may get resistant to currently available anti-cancer treatments [42].
CONCLUSION:
As a conclusion, here in this study we observed that ginger extract not only has a powerful cytotoxic effect on breast and pancreatic cancer cells’ proliferation in a dose- and time-dependent manner but also increased the level of p53 protein and on the other hand, did not significantly affect viability of normal cells. Since that selectivity for cancerous cells, high tolerance by humans and low toxicity for normal cells are ideal features of potential cancer chemopreventive and therapeutic agents, after more animal studies and human trials on ginger extract (especially using the purified active compounds), it can be considered as a novel chemopreventive and therapeutic agents. In addition, except for a few clinical studies in human subjects, most of the ginger components’ activities are according to in vitro and in vivo studies. Accordingly, further studies are required in this regard to show its efficacy as an anticancer agent, as a safe and cost-effective alternative.
Authors’ contribution:
SS and ZM conducted the research. MD and KG designed and supervised the study, prepared the final draft of the article.
ACKNOWLEDGMENTS:
This study was approved by the Aja University of Medical Sciences, Tehran, Iran and the authors accordingly would like to thank the University for the financial support.
Conflicts of interest:
The authors declare no conflict of interest.
Ethical considerations:
The authors have observed all ethical issues including plagiarism, misconduct, data fabrication, falsification, double publication or submission, and redundancy.
Funding/Support:
This study was supported by Aja University of Medical Sciences.
REFERENCES
- Hanahan, D. and R.A. Weinberg, Hallmarks of cancer: the next generation. Cell, 201 144(5): p. 646-74.
- Hassan, M., et al., Apoptosis and molecular targeting therapy in cancer. Biomed Res Int, 2014. 2014: p. 150845.
- Thun, M.J., et al., The global burden of cancer: priorities for prevention. Carcinogenesis, 2010. 31(1): p. 100-10.
- Salehiniya, H., et al., Time Trend Analysis of Cancer Incidence in Caspian Sea, 2004 - 2009: A Population-based Cancer Registries Study (northern Iran). Caspian J Intern Med, 2016. 7(1): p. 25-30.
- Rojas, K. and A. Stuckey, Breast Cancer Epidemiology and Risk Factors. Clin Obstet Gynecol, 2016. 59(4): p. 651-672.
- Jin, X. and P. Mu, Targeting Breast Cancer Metastasis. Breast Cancer (Auckl), 2015. 9(Suppl 1): p. 23-34.
- Youlden, D.R., et al., Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med, 2014. 11(2): p. 101-15.
- Miller, T.W., J.M. Balko, and C.L. Arteaga, Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol, 2011. 29(33): p. 4452-61.
- Klint, A., et al., Trends in survival of patients diagnosed with cancer of the digestive organs in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol, 2010. 49(5): p. 578-607.
- Beger, H.G., et al., Intraarterial adjuvant chemotherapy after pancreaticoduodenectomy for pancreatic cancer: significant reduction in occurrence of liver metastasis. World J Surg, 1999. 23(9): p. 946-9.
- Siegel, R., C. Desantis, and A. Jemal, Colorectal cancer statistics, 2014. CA Cancer J Clin, 2014. 64(2): p. 104-17.
- Ferlay, J., et al., Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 20 Int J Cancer, 2015. 136(5): p. E359-86.
- Rahib, L., et al., Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res, 2014. 74(11): p. 2913-21.
- Dorai, T. and B.B. Aggarwal, Role of chemopreventive agents in cancer therapy. Cancer Lett, 2004. 215(2): p. 129-40.
- Kaur, M., C. Agarwal, and R. Agarwal, Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J Nutr, 2009. 139(9): p. 1806S-12S.
- Kolonel, L.N., et al., Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol Biomarkers Prev, 2000. 9(8): p. 795-804.
- Surh, Y.J., Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer, 2003. 3(10): p. 768-80.
- Srinivasan, K., Antioxidant potential of spices and their active constituents. Crit Rev Food Sci Nutr, 2014. 54(3): p. 352-72.
- Shukla, Y. and M. Singh, Cancer preventive properties of ginger: a brief review. Food Chem Toxicol, 2007. 45(5): p. 683-90.
- Maghbooli, M., et al., Comparison between the efficacy of ginger and sumatriptan in the ablative treatment of the common migraine. Phytother Res, 2014. 28(3): p. 412-5.
- Mashhadi, N.S., et al., Anti-oxidative and anti-inflammatory effects of ginger in health and physical activity: review of current evidence. Int J Prev Med, 2013. 4(Suppl 1): p. S36-42.
- Raal, A., et al., Complementary treatment of the common cold and flu with medicinal plants--results from two samples of pharmacy customers in Estonia. PLoS One, 2013. 8(3): p. e58642.
- Al-Nahain, A., R. Jahan, and M. Rahmatullah, Zingiber officinale: A Potential Plant against Rheumatoid Arthritis. Arthritis, 2014. 2014: p. 159089.
- Koo, S.W., M.K. Lim, and K.W. Lee, Immunomodulatory effects of zingiber officinale in cyclophosphamide-induced immunosuppress mice. Journal of Veterinary Clinics, 2015. 32: p. 56-61.
- Seeram, N.P., et al., Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. J Agric Food Chem, 2004. 52(9): p. 2512-7.
- Nigam, N., et al., Induction of apoptosis by [6]-gingerol associated with the modulation of p53 and involvement of mitochondrial signaling pathway in B[a]P-induced mouse skin tumorigenesis. Cancer Chemother Pharmacol, 2010. 65(4): p. 687-96.
- Seemann, S., et al., The tumor suppressor gene TP53: implications for cancer management and therapy. Crit Rev Clin Lab Sci, 2004. 41(5-6): p. 551-83.
- Willis, A.C. and X. Chen, The promise and obstacle of p53 as a cancer therapeutic agent. Curr Mol Med, 2002. 2(4): p. 329-45.
- Xin, Y., et al., Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol Med, 2017. 14(3): p. 228-241.
- Elmore, S., Apoptosis: a review of programmed cell death. Toxicol Pathol, 2007. 35(4): p. 495-516.
- Bertheau, P., et al., p53 in breast cancer subtypes and new insights into response to chemotherapy. Breast, 2013. 22 Suppl 2: p. S27-9.
- Yin, S.Y., et al., Therapeutic applications of herbal medicines for cancer patients. Evid Based Complement Alternat Med, 2013. 2013: p. 302426.
- Rahimi Babasheikhali, S., S. Rahgozar, and M. Mohammadi, Ginger extract has anti-leukemia and anti-drug resistant effects on malignant cells. J Cancer Res Clin Oncol, 2019. 145(8): p. 1987-1998.
- Prasad, S. and A.K. Tyagi, Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract, 2015. 2015: p. 142979.
- Karna, P., et al., Benefits of whole ginger extract in prostate cancer. Br J Nutr, 2012. 107(4): p. 473-84.
- Pashaei-Asl, R., et al., The Inhibitory Effect of Ginger Extract on Ovarian Cancer Cell Line; Application of Systems Biology. Adv Pharm Bull, 2017. 7(2): p. 241-249.
- Hessien, M., et al., Growth inhibition of human non-small lung cancer cells h460 by green tea and ginger polyphenols. Anticancer Agents Med Chem, 2012. 12(4): p. 383-90.
- Sreedhar, A., J. Li, and Y. Zhao, Next-Gen Therapeutics for Skin Cancer: Nutraceuticals. Nutr Cancer, 2018. 70(5): p. 697-709.
- Nelson, W.G. and M.B. Kastan, DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol, 1994. 14(3): p. 1815-23.
- Zhang, S., et al., Zerumbone, a Southeast Asian Ginger Sesquiterpene, Induced Apoptosis of Pancreatic Carcinoma Cells through p53 Signaling Pathway. Evid Based Complement Alternat Med, 2012. 2012: p. 936030.
- Nalbantsoy, A., et al., Antimicrobial and Cytotoxic Activities of Zingiber officinalis Extracts. Journal of Pharmacy and Pharmaceutical Sciences, 2008. 33: p. 78-85.
- Park, Y.J., et al., [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. Yonsei Med J, 2006. 47(5): p. 688-97.
- El-Ashmawy, N.E., et al., Ginger extract adjuvant to doxorubicin in mammary carcinoma: study of some molecular mechanisms. Eur J Nutr, 2018. 57(3): p. 981-989.
- Hostanska, K., et al., Cimicifuga racemosa extract inhibits proliferation of estrogen receptor-positive and negative human breast carcinoma cell lines by induction of apoptosis. Breast Cancer Res Treat, 2004. 84(2): p. 151-60.
- Habib, S.H., et al., Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics (Sao Paulo), 2008. 63(6): p. 807-13.
- Levine, M.E., et al., Protein and ginger for the treatment of chemotherapy-induced delayed nausea. J Altern Complement Med, 2008. 14(5): p. 545-51.
- Citronberg, J., et al., Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: results from a pilot, randomized, and controlled trial. Cancer Prev Res (Phila), 2013. 6(4): p. 271-81.
- Sung, B., et al., Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res, 2008. 68(21): p. 8938-44.
- Elbendary, A.A., et al., Relationship between p21 expression and mutation of the p53 tumor suppressor gene in normal and malignant ovarian epithelial cells. Clin Cancer Res, 1996. 2(9): p. 1571-5.
- Elkady, A.I., et al., Differential control of growth, apoptotic activity, and gene expression in human breast cancer cells by extracts derived from medicinal herbs Zingiber officinale. J Biomed Biotechnol, 2012. 2012: p. 614356.
- Gaus, K., et al., Standardized ginger (Zingiber officinale) extract reduces bacterial load and suppresses acute and chronic inflammation in Mongolian gerbils infected with cagAHelicobacter pylori. Pharm Biol, 2009. 47(1): p. 92-98.
- Oyagbemi, A.A., A.B. Saba, and O.I. Azeez, Molecular targets of [6]-gingerol: Its potential roles in cancer chemoprevention. Biofactors, 2010. 36(3): p. 169-78.
