Archive \ Volume.11 2020 Issue 2
Evaluation of antibacterial spectrum and phytochemical analysis of Laurus nobilis Leaves extracts
Nehad M. Gum Gumjee
Department of Biology, College of Sciences, University of Jeddah, Jeddah, Saudi Arabia.
Abstract
Laurus nobilis is used as medicine and food additives in Saudi Arabia, and also, it could be used to study as antibacterial activity and assessment through agar well diffusion and most susceptible bacteria used was Pseudomonas aeruginosa, flowed by Staphylococcus aureus. Resistant bacteria mostly were Bacillus subtilis followed by MRSA while there was no effect on Micrococcus luteus. HPLC analysis found the presence of 11 phenolic compounds isolated from Laurus nobilisincluding Gallic Acid, Catechin, Coffeic Acid, Syringic Acid, Rutin, Coumaric Acid, Naringenin, Quercetin, Ferulic Acid, Vanillin, and Cinnamic Acid. The result showed that obtained found variations from the concentration ratio of ethanolic Laurus nobilis leaves extract. HPLC illustrated that Rutin, Gallaic acid, and syringic acid in high concentrations were 418.37, 81.66, and 34.43 µg/g, respectively and phenolic compounds ranged from 0.21 to 21.42 µg/g and the lowest Cinnamic concentration was 0.21µg/g.
Keywords: Laurus nobilis, Antibacterial, phytochemical constituents
INTRODUCTION
Resistance microorganism causes diseases that have posed a hazard in human and threatens public health. Many studies started in search of new alternatives to prevent disease [1, 2]. Plants consume a rich source of antimicrobial, as also have natural protection products against microbial occurrence [3-5].Laurel (L.) nobilis is an aromatic and commonly used culinary spice in Western and Asian countries such as Saudi Arabia. Cultivation of such a plant was in the Mediterranean and many warm countries. The aromatic tree is 2 m to 10 m high [6]. Leaves and berries are mainly used as sauce and spice aroma [7]. Laurus nobilisleaves are used to treat diseases. Essential oils and organic acids in this plant have shown strong antibacterial action against pathogenic strains [7-10]. The leaves have been examined ingredients. The Laurus nobili scomposition and yield are influenced by different factors, including extraction method, plant parts, yield season, growth environment, etc. In the present study, the ethanolic extract of Laurus nobilis leaves was examined using HPLC. This work will help to identify the phenolic compounds, which can be used in beneficial values. This study aimed at evaluating the chemical composition and antibacterial spectrum of Laurus nobilisleaves extract in Saudi Arabia.
MATERIALS AND METHODS
Collection and preparation of samples
Samples of Laurus nobilisleaves were collected during May 2019 from Albahahrigon (19°98' 28°N, 41°52' 50°E) southwest Saudi Arabia from cool slopes at 2050 m.a.s.l) Saudi Arabia, the species status of this plant was fervid at the faculty of Sciences Herbarium, King Abdulaziz University, Jeddah. The plant leaves were transported to the laboratory, washed in running tap water to eliminate dust particles and debris and then washed in distilled water for five minutes.
Preparation of Laurus nobilis leaves extracts
Ten grams of dried Laurus nobilisleaves washed to cutting to small pieces (1-2 mm) and adding 100 ml of distilled water and ethanol extract (1:10W/V)/48h and filtration later. Solutions evaporated with pressure (40°C) until dryness and diluted by dimethyl sulfoxide (DMSO) and stored at 20°C [11].
Phytochemical screening
Phenolic extraction
Phenolic extraction was conducted according to Mattila et al. [12]. 15 ml of4NNaOHwas added to 0.2L of water extract in a 50-ml Pyrex centrifuge tube purged with nitrogen and shaken in the dark for 2h with a wrist-action shaker. After phenolic acids liberated by alkaline hydrolysis, the samples were acidified with ice-cold HC1 (6N) to decrease pH to 1-2. Then the solution was centrifuged at 3000g and the supernatant was decanted into a 250-ml separator/funnel. The supernatant was extracted with ethyl acetate (3×50ml) by shaking for 10s and the mixture was allowed to settle for 5min between extractions. Ethyl acetate fractions were collected and pooled. The second supernatant was re-extracted with ethyl acetate (3×50ml) as before and all ethyl acetate fractions were pooled.
HPLC Analysis
Phenolic was separated according to Shimaduz (Kyoto, Japan) HPLC apparatus (model, LC-4A) equipped with visible/UV detector (model, SPD-2AS) at 280 nm and stainless steel column (25.0cm × 4.6mm i.d.).
Antibacterial Assay
Three Gram-negativebacteria: Escherichia coli (ATCC8739, Klebsiella pneumonia (ATCC700603), and Pseudomonas aeruginosa (ATCC27853) and four Gram-positive bacteria: Bacillus subtilis (ATCC11774); Methicillin-resistant Staphylococcus aureus (MRSA) (ATCC977), S.aureus (ATCC29213), and Micrococcus luteus (ATCC4698) are used to evaluate the efficacy of the extract. The tested strains were subcultured in nutrient agar medium slopes. The stock cultures were stored at 4°C.
Antibacterial Activity
The activity of bacterial strains was determined using the agar well diffusion assay methods according to Holder and Boyce [13]. DMSO was used as the negative control and streptomycin and ciprofloxacin (10 mg/disc) were used as the positive controls. Bacterial cultures were incubated at 37°C for 24h. Antimicrobial was determined by measuring the zone of inhibition [14].
Statistical Analysis
Means of variable and standard error were accepted using SPSS to detect any significant differences between pathogenic microorganisms and extract type.
RESULTS AND DISCUSSION
Antibacterial influence of the ethanolic extracts of Laurus nobilisleaves using disc diffusion against 4 Gram-positive bacteria andthree Gram-negative bacteria at 200 mg/ml, Laurus nobilisleaves extract given high activities against the tested organisms. The most susceptible strain was P. aeruginosa followed by S.aureus,while the most resistant strain was Bacillus subtilis followed by MRSA and the diameter of inhibition zones against bacteria was 29.00mm, 28.00mm, 17.00mm, and 18.00mm, respectively (Table 1). This study was consistent with the study conducted by Ramos et al. [15] in which the antibacterial effect of different solvent extracts and separated ingredients of Laurus nobiliswere evaluated and showed strong antibacterial action with the ethanol extract against pathogenic bacteria and foodborne spoilage .Noriakietal[16] found antimicrobial activity against many pathogens including opportunistic Gram-positive bacteria. In addition, the present results are in line with Al-Hussaini andAdel. [17] who showed that the leave extract of L. nobilis was additionally active against B.subtilis and P. aeruginosa in contract to Gram-negative bacteria. Also, Erturk[18] described eleven ethanolic extracts of spices for in vitro antibacterial effect against Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, and Bacillus subtilis, which was in line with the current results. Antimicrobial activities of standard antibiotics (Streptomycin (10 μg/disc) and Ciprofloxacin (10μg/disc)) had an inhibitory effect against all the tested bacteria. Ciprofloxacin is more effective than streptomycin (Table 1). Antimicrobial activity of Laurus nobilisleaves in terms of inhibition zone (mm) of the ethanolic extracts were tested at the concentration of 0.5 mg/ml against microorganisms. Laurus nobilis extract leaves has antimicrobial action against P. aeruginosa, S.aureus, Klebsiella pneumonia, and E. coli with inhibition zone of 29, 28, 26, and 24 mm, respectively, which was considerably higher than that of the control (Streptomycin) with the inhibition zones of 22, 19, 25, and 23 mm, respectively and no inhibition zone against Micrococcus strain that was parallel with Shital [19]. Inhibition diameters against bacteria are shown in Table (1). EL Malti and Amarouch[20]reported the average inhibition zone of L. nobilisleaves extract ranged from 7mm (Pseudomonas aeruginosa ATCC 27853) to 20mm (L. monocytogenes). The results presented antibacterial use of the bay extract for the treatment of S. aureus infection [21] and on human pathogenic bacteria by disc diffusion method via the average inhibition zone against 9 bacteria strains. L. nobilis effects on bacteria more than that of tetracycline antibiotic. Extract of L. nobilis presented strong anti-bacterial activity similar to the finding of Moghtader and Farahmand [22].
|
Table 1. The antibacterial activity of ethanolic extracts of Laurus nobilisleaves compared to antibiotics against different pathogenic bacteria. |
||||||
|
Diameter of the inhibition zone (mm) Mean ± SD |
|
|||||
|
Bacterial strains |
Laurus nobilis leaves extracts |
Streptomycin (10 μg/disc) |
Ciprofloxacin (10μg/disc) |
|
||
|
Bacillus subtilis |
|
25.00±00.00 |
34.00±00.00 |
|
||
|
MRSA |
|
20.00±00.00 |
36.00±00.00 |
|
||
|
Micrococcus luteus |
00.00±00.00 |
27.00±00.00 |
46.00±00.00 |
|
||
|
Staphylococcus aureus |
28.00±00.00 |
19.00±00.00 |
38.00±00.00 |
|
||
|
Klebsiella pneumonia |
26.00±00.00 |
25.00±00.00 |
42.00±00.00 |
|
||
|
E. coli |
24.67±0.58 |
23.00±00.00 |
44.00±00.00 |
|
||
|
Pseudomonas aeruginosa |
29.00±00.00 |
22.00±00.00 |
42.00±00.00 |
|
||
Phytochemical Screening of. Laurus nobilis
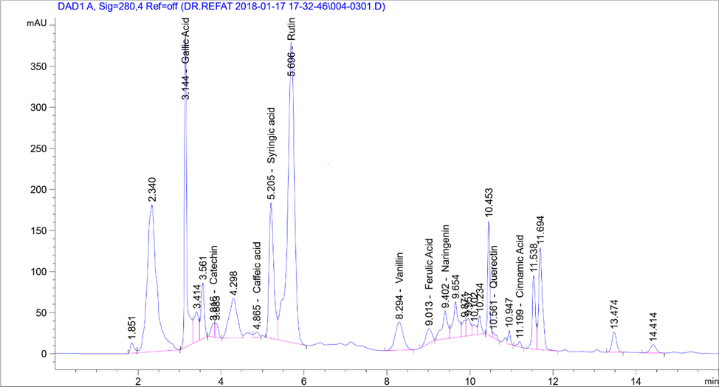
HPLC analysis found the presence of 11 phenolic compounds isolated from Laurus nobilis including Quercetin, Rutin,Syringic Acid, Coffeic Acid, Catechin, Gallic Acid, Coumaric Acid, Vanillin, Ferulic Acid, Naringenin, and Cinnamic Acid). HPLC showed Rutin, Gallaic acid, and syringic acid at high concentrations of 418.37, 81.66, and 34.43µg/g, respectively. Phenolic compounds' concentrations ranged from 0.21 to 21.42 µg/g and Cinnamic Acid had the minimum concentration (0.21µg/g) (Table 2).
These results are consistent with the results of Stefano et al. [23] who showed that phytochemicals had many flavonoid derivatives. Semipreparative HPLC of laurel leaves found 10 flavonoid O-glycosides, under the best extraction by Muñiz-Márquez et al. [24]. Active principles identified showed antimicrobial activity against test organisms parallel to the observations of Gumgumje et al. [25], Gumgumjee& Hajar [26], and Hajar & Gumgumjee [27]. The extracts from various parts of L. nobilisconsisted of 1, 8 Cineole, sabinene, α-pinene, and p-Cymene and many biological and pharmacological characterization recorded the antibacterial effect of L. nobilis leave extract [28]. These results are consistent with the results by Muñiz-Márqueza et al. [29] in which the phytochemical screening by HPLC analysis determined 4 phenolic compounds in the extract: coumaric, gallic, pyrogallol and resorcinol. Further studies involved in each compound are required. Previous studies established the presence of other phenolic compounds in L. nobilis. Muñiz-Márqueza et al. [30] documented the presence of caffeic, vanillic, and ferulic acids. Lu et al. [31] found rutinin and unknown phenolic acids. Environmental conditions had effects on phenolic compounds in plants [32, 33]. Chemical analysis of L. nobilis extract showed the presence of alkaloids, flavonoids, tannins, and essential oil [34]. In addition, a study by Ramling et al. [35], showed that Laurus nobilisleaves produced four nonpolar flavonoids kaempferol-3-O-α-L-(3",4"-di-E-p-coumaroyl)- rhamnoside, kaempferol-3-O-α-L-(2",4"-di-E-pcoumaroyl)-rhamnoside, kaempferol-3-Oα-L-(2"-4"-pcoumaroyl)- rhamnoside, and a new product kaempferol-3-O-α-L-(2",4"-diZ-p-coumaroyl)-rhamnoside[36]. 5 discovered megastigmane glucosides called laurosides A−E were isolated as novel phenolic glucoside from methanolic extract of L. nobilisL. leaves. Kaempferol- 3-rhamnopyranoside,andkaempferol-3, 7- di-rhamnopyranoside isolated fromLaurus nobilisaqueous and ethanolic extracts [37] (Table 2).
|
Table 2: Chemical composition analysis of Laurus nobilis leave extracts. |
||
|
Phenolic compounds |
Area |
Conc. (µg/ml) |
|
Gallic Acid |
1502.94 |
81.66 |
|
Catechin |
93.90 |
21.42 |
|
Coffeic Acid |
52.51 |
1.33 |
|
Syringic Acid |
1308.59 |
34.43 |
|
Rutin |
4245.16 |
418.37 |
|
Coumaric Acid |
0.00 |
0.00 |
|
Vanillin |
464.16 |
8.87 |
|
Ferulic Acid |
178.52 |
2.85 |
|
Naringenin |
324.70 |
12.14 |
|
Querectin |
41.31 |
1.28 |
|
Cinnamic Acid |
34.38 |
0.21 |
CONCLUSION
High differences in volatile components of the ethanolic extract of Laurus nobilis leaves are related to diverse geographic origins, growing conditions, periodic conditions, and procedures. Major compounds in Laurus nobilis leaves were Gallic acid, catechin, coffeic acid, syringic acid, Rutin, coumaric acid, vannillin, ferulic acid, naringenin, Quercetin, and cinnamic acid. The leaf of Laurus nobilishad high pharmacological actions such as antimicrobial and a variety of constituents in that could be responsible for an extensive range of biological activities of the plant.
REFERENCES
- Ushimaru PI, Silva MT, Di Stasi LC, Barbosa L, Fernandes Junior A. Antibacterial activity of medicinal plant extracts. Brazilian Journal of Microbiology. 2007 Dec;38(4):717-9.
- Usman A, Ming LC, Khan TM. Drug-Resistant Sexually Transmitted Infections in Southeast Asia: Regional Challenges to Control. Archives of Pharmacy Practice. 2019 Jan 1;1:1.
- Kosikowska U, Smolarz H, Malm A. Antimicrobial activity and total content of polyphenols of Rheum L. species growing in Poland. Cent.Eur.J.Biol. 2010; 10 : 65- 69.
- Thanish Ahamed S, Lakshmi T. Antibacterial Activity of Taxifolin Isolated from Acacia Catechu Leaf Extract–An in Vitro Study. International Journal of Pharmaceutical Research & Allied Sciences. 2018 Oct 1;7(4).
- Peña JF, Dapar ML, Aranas AT, Mindo RA, Cabrido CK, Torres MA, Manting MM, Demayo CG. Assessment of antimicrobial, antioxidant and cytotoxic properties of the ethanolic extract from Dracontomelon dao (Blanco) Merr. & Rolfe. Pharmacophore.. 2019 Jun 1;10:18-29.
- Dall'Acqua S, Viola G, Giorgetti M, Loi MC, Innocenti G. Two new sesquiterpene lactones from the leaves of Laurus nobilis. Chemical and pharmaceutical bulletin. 2006;54(8):1187-9.
- Yilmaz ES, Timur M, Aslim B. Antimicrobial, antioxidant activity of the essential oil of Bay Laurel from Hatay, Turkey. Journal of Essential Oil Bearing Plants. 2013 Feb 1;16(1):108-16.
- Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils–a review. Food and chemical toxicology. 2008 Feb 1;46(2):446-75.
- Ozogul I, Polat A, Özogul Y, Boga EK, Ozogul F, Ayas D. Effects of laurel and myrtle extracts on the sensory, chemical and microbiological properties of vacuum‐packed and refrigerated European eel (A nguilla anguilla) fillets. International journal of food science & technology. 2014 Mar;49(3):847-53., Doi :10.1111/ijfs.12374
- Rafiq R, Hayek SA, Anyanwu U, Hardy BI, Giddings VL, Ibrahim SA, Tahergorabi R, Kang HW. Antibacterial and Antioxidant Activities of Essential Oils from Artemisia herba-alba Asso., Pelargonium capitatum× radens and Laurus nobilis L. Foods. 2016 Jun;5(2):28.
- Boeru V, Derevici A. Some chemical and physical data on Romania propolis. Apimondia" propolis" Bucharest. 1978:19-26.
- Mattila P, Pihlava JM, Hellström J. Contents of phenolic acids, alkyl-and alkenylresorcinols, and avenanthramides in commercial grain products. Journal of Agricultural and Food Chemistry. 2005 Oct 19;53(21):8290-5.
- Holder IA, Boyce ST. Agar well diffusion assay testing of bacterial susceptibility to various antimicrobials in concentrations non-toxic for human cells in culture. Burns. 1994 Oct 1;20(5):426-9.
- Agwa A, Aly MM, Bonaly R. Isolation and characterization of two Streptomyces species produced non polyenic antifungal agents. J. Union Arab Biol. 2000;7:62-84.
- Ramos C, Teixeira B, Batista I, Matos O, Serrano C, Neng NR, Nogueira JM, Nunes ML, Marques A. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Natural product research. 2012 Mar 1;26(6):518-29.
- Fukuyama N, Ino C, Suzuki Y, Kobayashi N, Hamamoto H, Sekimizu K, Orihara Y. Antimicrobial sesquiterpenoids from Laurus nobilis L. Natural product research. 2011 Aug 1;25(14):1295-303.
- Al-Hussaini R, Mahasneh AM. Antibacterial and antifungal activity of ethanol extract of different parts of medicinal plants in Jordan. Jordan Journal of Pharmaceutical Sciences. 2011;4(1):57-69.
- Ertürk Ö. Antibacterial and antifungal activity of ethanolic extracts from eleven spice plants. Biologia. 2006 Jun 1;61(3):275-8.
- Shital S. Characterization of Some Antimicrobial Substances from Seed Coat of Tamarindus indica L. Br. J. Pharmacol. Toxicol. 2010;1(1):29-32..
- El Malti J, Amarouch H. Antibacterial effect, histological impact and oxidative stress studies from Laurus nobilis extract. Journal of food quality. 2009 Apr;32(2):190-208.
- Ghadiri E, Ahmadi R, Moridikyia A, Mahdavi E, Tavakoli P. Laurusnobilis Has Antibacterial Activity Against Staphylococcus aureus. InInternational Conference on Food, Biological and Medical Sciences, Jan 2014 (pp. 28-29).
- Moghtader M, Farahmand A. Evaluation of the antibacterial effects of essential oil from the leaves of Laurus nobilis L. Kerman Province//J. Microbiology and Antimicrobials. 2013;5(2):13-7.
- Dall'Acqua S, Cervellati R, Speroni E, Costa S, Guerra MC, Stella L, Greco E, Innocenti G. Phytochemical composition and antioxidant activity of Laurus nobilis L. leaf infusion. Journal of medicinal food. 2009 Aug 1;12(4):869-76.
- Muñiz-Márquez DB, Rodríguez R, Balagurusamy N, Carrillo ML, Belmares R, Contreras JC, Nevárez GV, Aguilar CN. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CyTA-Journal of Food. 2014 Jul 3;12(3):271-6.
- Gumgumjee NM, Hajar AS. Antimicrobial efficacy of Casuarina equisetifolia extracts against some pathogenic microorganisms. Journal of Medicinal Plants Research. 2012 Dec 10;6(47):5819-
- Gumgumjee NM, Khedr A, Hajar AS. Antimicrobial activities and chemical properties of Tamarindus indica L. leaves extract. African journal of microbiology Research. 2012 Aug 23;6(32):6172-81.
- Hajar, A. S., Gumgumjee, N. M. Antibacterial Efficiency and DNA Impairment Unveil in some Bacteria strains treated with Conocarpus erectus L. extract. International Journal of Applied biology and Pharmaceutical Technology-(IJABPT), 2013; (4):37-47.
- Chahal KK, Kaur M, Bhardwaj U, Singla N, Kaur A, Kaur M, Bhardwaj U, Singla N, Kaur A. A review on chemistry and biological activities of Laurus nobilis L. essential oil. Journal of Pharmacognosy and Phytochemistry. 2017;6(4):1153-61.
- Muñiz-Márquez DB, Rodríguez R, Balagurusamy N, Carrillo ML, Belmares R, Contreras JC. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L. and Amaranthus hybridus L. CyTA-Journal of Food. 2014 Jul 3;12(3):271-6.
- Muñiz-Márquez, R. Rodríguez, N. Balagurusamy, M.L. Carrillo, R.Belmares, J.C. Contreras, G.V. Nevárez, Aguilar, C. N. Phenolic content and antioxidant capacity of extracts of Laurus nobilis L., Coriandrum sativum L.and Amaranthus hybridusL.cyta journal of food, 2007; 12:3, 271-276.
- Lu M, Yuan B, Zeng M, Chen J. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Research International. 2011 Mar 1;44(2):530-6.
- Nana FW, Hilou A, Millogo JF, Nacoulma OG. Phytochemical composition, antioxidant and xanthine oxidase inhibitory activities of Amaranthus cruentus L. and Amaranthus hybridus L. extracts. Pharmaceuticals. 2012 Jun;5(6):613-28.
- Upadrasta, L., Mukhopadhyay, M., Banerjee, R. Chemistry and biotechnology of polyphenols. Tannins: Chemistry, biological properties and biodegradation. Kerala, India: CIBET Publishers, 2011.
- Trease G. E., Evans WC. Pharmacognosy. 11th edition. BrailliarTiridal Can Macmillian Publishers; 2008.
- Rampling J, Mitchell AJ, Von Oertzen T, Docker J, Jackson J, Cock H, Agrawal N. Screening for depression in epilepsy clinics. A comparison of conventional and visual‐analog methods. Epilepsia. 2012 Oct;53(10):1713-21.
- Fiorini C, David B, Fourasté I, Vercauteren J. Acylated kaempferol glycosides from Laurus nobilis leaves. Phytochemistry. 1998 Mar 1;47(5):821-4.
- De Marino S, Borbone N, Zollo F, Ianaro A, Di Meglio P, Iorizzi M. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. Journal of agricultural and food chemistry. 2004 Dec 15;52(25):7525-31.
