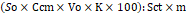Archive \ Volume.14 2023 Issue 4
Metafiltrate-Based Dietary Supplement to Improve Gut Biocenosis and Support Metabolic Detoxification
Maria Anatolevna Zakharenko1, Valeriy Mikhailovich Poznyakovsky1,2, Lapina Valentina3*, Ulubieva Elena Arsenovna4, Kulova Laura Alexandrovna4, Loseva Anna Ivanovna5
1Department of Technological Entrepreneurship, Kuzbass State Agricultural Academy, Kemerovo, Russia. 2Scientific and Educational Center for Applied Biotechnology and Nutrition, Kemerovo State Medical University, Kemerovo, Russia. 3Department of Management, Entrepreneurship and Engineering, Ural State University of Economics, Ekaterinburg, Russia. 4Department of Internal Diseases N1, North Ossetian State Medical Academy (SOGMA), Vladikavkaz, Russia. 5Laboratory of Biotesting of Natural Nutraceuticals, Kemerovo State University, Kemerovo, Kemerovo region – Kuzbass, Russia.
Abstract
The present study deals with a new highly effective functional product, a dietary supplement, to improve gut biocenosis and support metabolic detoxification. The supplement includes the following ingredients (mg per capsule): postbiotic metafiltrate -200; natural zeolite -130.36; polysorbovit – 105 (soluble dietary fiber – 100); silicon dioxide – 14.46; lipase – 10; bromelain – 10; papain – 10; cellulase – 10; rutin – 6; chitosan – 5; lysozyme hydrochloride – 5. The supplement is considered innovative as capsules have a special enteric coating to provide successive and targeted delivery of ingredients to the gastrointestinal tract cells while preserving safety and bioactivity. The antimicrobial and antifungal activity of the postbiotic metafiltrate has been studied. The mechanism of endotoxin neutralization and biocenosis normalization is characterized by the following factors: the destruction of toxic peptidoglycans of the bacterial cell wall and proteins and peptides with different chain lengths; elimination of Candida albicans cells by destroying bonds in the beta-glycan molecule of the fungal cell wall; cleavage of toxic lipid A of lipopolysaccharides; the influence of the culture fluid metabolites of probiotic microorganisms; high sorption capacity and selectivity for endotoxins, toxic metabolites, pathogenic microorganisms, free radicals, allergens, and antigens. The product has been included in the volunteers’ diet and its efficacy has been assessed. The product's role in metabolic detoxification and biocenosis normalization has been studied. The recommended dosage is 1 capsule twice a day with a meal for 30 days.
Keywords: Dietary supplement, Enzymes, Sorbents, Composition, Postbiotic metafiltrate, Metabolic detoxification
INTRODUCTION
Today there exist numerous experimental and clinical studies analyzing the role of the microbiome, including the gut microbiota, in maintaining health, development, and progression of different diseases [1-8].
The microbiome of a person is exposed to several negative factors: malnutrition, consequences of common digestive and infectious diseases, overuse of antibiotics and other medications, etc., which may lead to dysbacteriosis development and endotoxemia as a result [9, 10]. The main sources of endotoxins are lipopolysaccharides, beta-glycans of gram-negative pathogenic bacteria and fungi, and the cell wall peptidoglycans of gram-positive ones [11, 12]. These endotoxins are not destroyed by the gut enzyme system. They enter the bloodstream and cause various immunological reactions and aseptic systemic inflammation [13]. Leaky gut syndrome contributes to this when a lot of additional endotoxins enter the bloodstream through the intestinal wall as undigested protein residues, intensifying inflammation and causing intoxication [14-22].
Biotechnological products with microbiological profiles and targeted functional properties can be used as an effective way of metabolic detoxification and gut biocenosis normalization [23].
Stages of Drug Development
MATERIALS AND METHODS
The materials of the study are raw materials and product samples (industrial and laboratory). 60 volunteers with gut microbiota deviations were involved in the clinical trial to assess the efficacy of the product and determine its functional properties.
Quality characteristics of the product were tested according to the national standards recorded in the current regulatory documentation: yeasts and molds (10444.12-2013); coliforms (31747-2012); pathogens, including salmonella (31659-2012); E coli (30726-2001); QMAFAnM (10444.15-94); pesticides, sums of HCCH and DDT isomers, aldrin, heptachlor (30349-96); mercury (26927-86); cadmium and lead (30178-96); iron (4.1.1672-03).
Fermentation was used to study soluble dietary fiber. This method involves enzymatic hydrolysis of proteins and starches. In this case, the used enzymes are similar to those of the gastrointestinal tract (pancreatin, glucoamylase, protease, or pepsin A). Hydrolysis is followed by spectrometric registration (4.1.1572.03).
Rutin content was determined by high-performance liquid chromatography (HPLC) when rutin was extracted with ethanol and the subsequent quantitative analysis was carried out by HPLC.
Rutin quantitative analysis is conducted by using a test solution. For this purpose, a test sample, equivalent to 1 mg of flavonoid, is placed in a 100 cm3 flask, and then 80cm 3 of ethyl alcohol is added. After that, the flask is heated in a water bath at the solution boiling temperature for 60 minutes. Then the obtained filtrate is poured in a 100 cm3 flask, washed by adding successively 5 cm3 of distilled water and put in a volumetric flask. The solution should reach the mark. Finally, the obtained solution is stirred and filtered (filter, 0.45 μm).
A standard solution of the flavonoid under study is prepared: an exact weight of rutin (50 mg) is placed into a 100 cm3 flask, then ethyl alcohol is added, and the flask is heated in a water bath. The obtained solution is diluted 50-fold with ethyl alcohol and filtered (filter, 0.45 μm).
A mobile phase consisting of solution A (Н3РО4 рН 3.0) and solution B (Acetonitrile) is prepared. The solutions are first degassed and then filtered (filter, 0.45 μm).
The following HPLC parameters were used during the chromatographic analysis: internal column diameter – 3.9 mm, pre-column length – 20 mm (diameter – 3.9 mm), and column length – 150 mm. Symmetry C18 (5 μm) was used in the stationary phase. A UV detector (255 nm, 370 nm) was utilized. The work sample volume was 10 mcL, and the chromatogram retention time was 15 minutes.
Calculation of the research results: after preparing the chromatography line, we started injecting 10 μm of test and standard samples into the column. The obtained chromatograms are analyzed by measuring the peak areas. Rutin is calculated by the formula:
|
So×Ccm×Vo×K×100:Sct×m |
(1) |
where:
So – peak area of the test sample;
Sсt – peak area of the standard sample;
Ссm – concentration of the standard sample solution, mg/mL;
Vo – volume of the test solution, mL;
k - dilution factor;
m – sample weight, mg.
The method for assessing the bactericidal and bacteriostatic activity of the product-immobilized metafiltrates against gram-negative bacteria was developed and tested, using E. coli as an example. The results are presented in Table 1.
The obtained materials were analyzed 24 hours later while comparing the control and antimicrobial samples by microorganisms’ growth. The percentage of dead test microorganisms was calculated.
Having measured the bacteria level in control samples before and after incubation, we were able to assess bactericidal and bacteriostatic activity.
|
Table 1. Bactericidal and bacteriostatic activity of the product immobilized meta filtrates against gram-negative bacteria, E. coli |
|||||
|
Samples |
Parameter |
Viable bacteria cell count, CFU/ml |
Common logarithm of the number of bacteria (Lg) |
Bacteriostatic activity (S) Mb - Mc |
Bactericidal activity (L) Ma - Mc |
|
Zeolite (control) |
Mb |
3.3·105 |
5.52 |
- |
- |
|
Zeolite + metafiltrates of probiotic bacteria |
Mc |
2.0·102 |
2.30 |
3.22 |
0.24 |
The formula used for calculation:
S = Mb – Mc;
L = Ma – Mc, where:
S – bacteriostatic activity indicator;
L – bactericidal activity indicator;
Ма - logarithm of bacteria number at the end of inoculation process, the active agent is not taken into account;
Mb - logarithm of bacteria number after 24 hours of incubation, active agent is not taken into account;
Mc - logarithm of bacteria number after 24 hours of incubation, the active agent is taken into account.
The bactericidal activity indicator is reliable when L is greater than zero.
The bacteriostatic activity indicator is reliable when S is greater than 2.
Thus, the metafiltrate immobilized on zeolite demonstrates bactericidal and bacteriostatic activity against gram-negative pathogenic and opportunistic microorganisms.
If compared to the control sample, the test sample inhibits the growth of microorganisms, with the number of bacteria decreasing 3-fold.
RESULTS AND DISCUSSION
An innovative biologically active dietary supplement was developed to effectively support metabolic detoxification and improve gut biocenosis. The study characterizes the product ingredients and determines its qualitative and quantitative composition and functional properties.
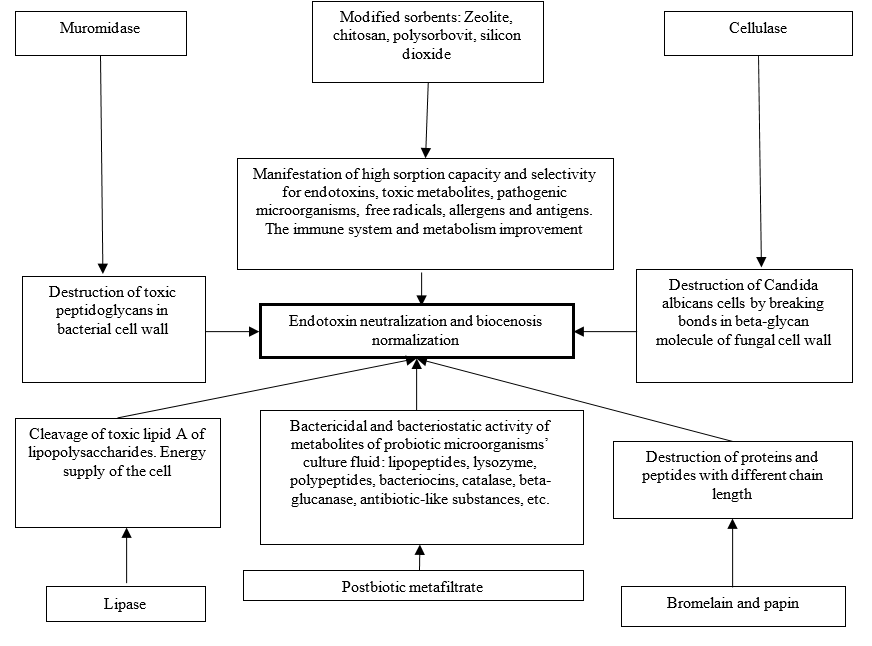
Postbiotic metafiltrate "Subtizym" contains a wide range of enzymes and antibacterial peptides active against gram-negative and gram-positive fungi and pathogens. The metabolic products of biologically active metabolites of probiotic microorganisms’ cell-free culture fluid include lysozyme, polypeptides, bacteriocins, catalases, lipopeptides, beta-glucanases, antibiotic-like substances, etc, which demonstrate bactericidal and bacteriological activity without affecting the gut beneficial bacteria.
Lysozyme (muromidase) is a protein enzyme (mucopeptide – glucohydrolase). It destroys toxic peptidoglycans of the bacterial cell wall and shows the greatest effectiveness in combination with probiotic bacteria metafiltrate. They are predominantly located in organs and tissues constantly exposed to undesirable microorganisms.
Lysozyme destroys murein (peptidoglycan). This activity is explained by the mechanism of hydrolysis of the glycosidic bond between N-acetylglucosamine and N-acetylmuramic acid. In this case, peptidoglycan is bound by the lysozyme active site in the cleft between its structural domains. Lysozyme has 6 binding pockets – A, B, C, D, E, F. Pockets B, D, and F bind both N-acetylglucosamine and N-acetylmuramic acid, while pockets A, C, and E only bind N-acetylglucosamine.
Cellulase is 1,4, 1,3 and 1,6 β-glucanase. This enzyme destroys 1,3 and 1,6 bonds specific for Candida albicans in the beta-glycan molecule of the fungal cell wall. This leads to cell membrane disruption and cell death. The enzyme is effective at different stages of fungal development.
Natural zeolite has certain advantages over other traditional sorbents because of its selectivity, which is provided by zeolite structural features and ion exchange. Zeolite microporous structure resembles a honeycomb with negatively charged silicon, potassium, and magnesium ions inside. So, zeolite attracts any positively charged toxic substances, e.g. ions of strontium, mercury, rubidium, lead, cesium, cadmium, ammonium, arsenic, bacterial endotoxins, proteins, etc., and exchanges them for its ions, thus performing its detoxifying function. Zeolite is also able to bind allergens and antigens causing allergies, asthma, and migraines, thus reducing their manifestation symptoms.
Chitosan is a universal sorbent capable of adsorbing different toxic substances of organic and inorganic nature, including undigested food, from the gut and blood. Chitosan detoxifies the body, has a positive impact on the mucous membrane of the gastrointestinal tract, improves the function of the kidneys, liver, and gut, and prevents allergies. Figuratively, it is responsible for the “purity” of the internal environment.
Low sorption of bacterial lipopolysaccharides (LPS) is explained by a slightly acidic medium on the zeolite surface caused by the decomposition of silanol groups. Chitosan and its amine groups modify the outer surface of zeolite and that results in significantly higher sorption (up to 99%) of the cell wall LPS of gram-negative bacteria. The obtained organomineral sorbent is effective in a wide pH range, binding both lipopolysaccharides of gram-negative bacteria and peptidoglycans of the cell wall of gram-positive bacteria.
Polysorbovit is modified pectin with a high degree of esterification. It possesses a remarkable LPS sorption capacity, so it is very effective for toxicosis and diseases of different natures, including viral and bacterial.
Silicon dioxide nanoparticles can bind numerous toxins and remove them from the body due to a relatively large sorption surface of 200 m2/g. The sorbent is nontoxic, does not affect the pH of the medium and desorption, and does not traumatize the gastrointestinal tract mucosa. At the same time, we observe good gut evacuation and high absorptive capacity.
Lipase is an enzyme that catalyzes the breakdown of insoluble esters – lipid substrates. Lipase is specific to A1, A2, or A3 fragments of the glycerin backbone of a lipid substrate. Together with bile, it is involved in breaking down fats and fatty acids followed by energy production in the form of ATP. The product composition provides for the breakdown of toxic lipid A lipopolysaccharide.
Bromelain and papain are enzymes of a wide proteolytic spectrum. They can break down proteins and peptides, regardless of the pH medium. That is why they are effective for treating digestive disorders and limiting the influence of dominant fungal flora. They can significantly reduce protein decay and sugar fermentation, thus preventing intoxication and minimizing dysbiosis. The dietary supplement enzymes are immobilized on the carrier, which enhances their safety and effectiveness.
The product composition is presented in Table 2.
|
Table 2. Product composition |
|
|
Ingredients |
Mg per capsule |
|
Postbiotic metafiltrate " Subtizym" |
200 |
|
Natural zeolite |
130.36 |
|
"Polysorbovit " Soluble dietary fiber |
105 100 |
|
Silicon dioxide (Neosyl GP) |
14.46 |
|
Lipase |
10 |
|
Bromelain |
10 |
|
Papin |
10 |
|
Cellulase |
10 |
|
Rutin |
6 |
|
Chitosan |
5 |
|
Lisozyme hydrochloride |
5 |
|
Talc (anti-caking agent) |
22.6 |
|
Hydroxypropyl methylcellulose (carrier) |
14.6 |
|
Calcium stearate (anti-caking agent) |
5.5 |
|
Polyethylene oxide (water retaining agent) |
1.48 |
|
Total content weight |
550 |
|
Gelatin (carrier) |
99.85 |
|
Glycerin (water retaining agent) |
0.15 |
|
Total hard gelatin capsule weight |
100 |
|
Kollicoat МАЕ 100Р (carrier) |
48.95 |
|
Propylene glycol (water-retaining agent) |
30 |
|
Talc (агент anti-caking agent) |
28.42 |
|
Kollidon (carrier) |
12.63 |
|
Total shell weight |
120 |
|
Total capsule weight |
770 |
We developed a manufacturing technology of a multi-structural encapsulated form of a dietary supplement, each product ingredient influencing its functional properties. The technology may be considered innovative due to a special enteric coating to ensures sequential delivery of biologically active ingredients to the target cells of the gastrointestinal tract while keeping the ingredients safe and biologically active.
The manufacturing technology includes the following stages:
- Raw ingredients are sieved through a 1 mm sieve. The obtained screenings are crushed in a hammer mill and sieved through a 1 mm sieve again.
- Semi-finished product № 1 is prepared by dosing postbiotic metafiltrate, talc, aerosil 380, and base layer together. The obtained homogeneous suspension is put on the measured amount of zeolite in the fluidized bed.
Grinding (regranulation) is carried out in a granulator (Fitz Mill) with mesh № 4. Dry granulate is sieved through a 1 mm Vibro sieve, crushed, and sieved again. The mixture is examined for lumps and inclusions;
- Semi-finished product № 2 is prepared by dosing zeolite and chitosan together. The obtained homogeneous mixture is crushed (dry granulation) in a hammer mill with mesh № 4;
- Preparing the powdering mixture is carried out by dosing polysorbovit, lipase, bromelain, papain, silicon dioxide, rutin, cellulase, lysozyme hydrochloride, talc, and calcium stearate together. The mixture is sieved through a 1 mm Vibro sieve and then it goes to a hammer mill and sieved again. The mixture is examined for lumps and inclusions. The powdering mixture is prepared in a V-shaped mixer for 60 minutes (per 100 kilos of mixture). Lumps and inclusions are not allowed;
- Preparing the encapsulation composition is performed in a V-shaped mixer, where semi-finished products 1 and 2, and the powdering mixture (total weight 100 kg) are mixed for 60 minutes. Storage period must be 15 days maximum;
- Encapsulation is performed with an automatic capsule filler ZANASI 40E. The average weight of capsules is controlled by weighing 20 capsules together and individual capsules every 30 minutes. The spread in values is ± 5 %. The capsule appearance is examined every 60 minutes for abrasion, dents, chips, etc. The obtained capsules are dedusted and film-coated;
- An acid-resistant film coating is prepared in a homogenizer reactor. The measured amount of kollicoat and water is mixed for 60 minutes, and then propylene glycol is added, while the stirrer is running. Talk, titanium dioxide, and kollidon are mixed in a separate container and sieved through a 1 mm sieve. Then small portions of the mixture are put into the homogenizer reactor together with the kollicoat solution, while the stirrer is working. Homogenization is performed for 1 minute. After that, the obtained suspension is sprayed on capsules, up to 120 mg per capsule, with a film coating machine.
Finished capsules that meet the requirements of the technical documentation are sent for packing, packaging, and storing.
The qualitative characteristics of the product were thoroughly tested after the storage period of 27 months. The regulated manufacturing parameters are as follows: humidity is a maximum of 60 %, and temperature is a maximum of 25 ºС.
The product safety criteria were studied; its microbiological and sanitary-toxicological indicators were tested according to the Technical Regulation of Customs Union 021/2011.
The results presented in Table 3 comply with the declared safety indicators.
A shelf life of 24 months was established.
The developed dietary product as part of a personified combined nutritional program to normalize intestinal dysbiosis was tested during the clinical trial. It involved volunteers with identified intestinal biocenosis disorders in combination with other diseases.
They took the dietary supplement one capsule twice a day with a meal for 45 days. At the same time, they took other con-meds to produce the synergistic effect on indigenous microflora.
|
Table 3. Results of microbiological studies |
|||
|
Indicator |
Value |
||
|
Permissible value |
Actual value |
||
|
KMAFanM, CFU/g, maximum |
1×104 |
Maximum 90 |
|
|
Yeasts and molds, CFU/g, maximum |
100 |
Maximum 15 |
|
|
В.cereus, CFU/g, maximum |
200 |
20 |
|
|
E CoLi |
1.0 |
Not detected |
|
|
S.aureus |
1.0 |
Not detected |
|
|
Pathogens, including salmonella |
10.0 |
Not detected |
|
|
Coliforms |
0.1 |
Not detected |
|
|
Content of toxic elements and pesticides |
|||
|
Toxic elements |
Lead |
6.0 |
1.93 |
|
Arsenic |
3.0 |
0.33 |
|
|
Cadmium |
1.0 |
0.024 |
|
|
Mercury |
1.0 |
0.026 |
|
|
Pesticides |
HCCH (Sum of isomers) |
0.05 |
0.005 |
|
DDT and its metabolites |
0.05 |
0.005 |
|
|
Heptachlor |
Maximum 0.002 |
0.002 |
|
|
Aldrin |
Maximum 0.002 |
0.002 |
|
Laboratory studies involved analyzing immunological, hematological, and biochemical blood parameters before and after treatment. In addition, the patients’ fecal sugar and occult blood tests, and comprehensive digestive stool analysis were done. All participants completed the questionnaire to subjectively evaluate their physical condition on a 10-point scale before and after the program. The evaluation results are presented in Table 4.
|
Table 4. Evaluation of physical condition before and after product intake |
||
|
Indicator |
Before treatment (points) |
After treatment (points) |
|
Condition of skin, hair, nails (hair loss, brittle nails) |
9 |
2 |
|
Condition of mouth, nose, pharynx (coated tongue, dry skin) |
6 |
1 |
|
Condition of the gastrointestinal tract (flatulence, rumbling) |
10 |
1 |
|
Stool characteristics (consistency, frequency, constipation) |
8 |
1 |
|
Condition of nervous system (weakness, fatigue, dizziness) |
10 |
0 |
|
Condition of the musculoskeletal system (pains, crepitus) |
6 |
4 |
This data confirms that the patient’s physical health improved (from 60 to 100%). We can also speak of their better psycho-emotional state.
All participants demonstrated positive dynamics of cytolysis, cholestasis, and lipid metabolism. Before the program, 60% of the volunteers had disaccharides in feces, after the program disaccharides were not detected at all.
When studying the microbial landscape of the participants before the program, we identified various changes in the intestinal microflora: pathogenic microorganisms – fungi Candida albicans, Klebsiella pneumoniae; reduction of beneficial symbionts - Escherichia coli (Lac+), lacto- and bifidobacteria. After the program, 90% of participants demonstrated normalized microbiota.
All patients showed positive changes in stool test results.
The participants of the 45-day diet therapy program reported no side effects.
To explain the positive effect of postbiotic metafiltrate on metabolic detoxification and biocenosis normalization it is worth considering the structural and functional properties of bacterial lipopolysaccharides.
LPS include O-antigen, central oligosaccharide, and lipid A, three covalently linked components.
O-antigen structure includes oligosaccharides linked with polysaccharide chains and depends on the species of the bacterial strain.
The polysaccharide chain length has a significant impact on the penetration of antibiotics into a bacterial cell. The longer the chain, the less the antibiotic effect. The O-antigen of LPS is easily recognized by the immune system, so it is the most immunogenic component.
Central oligosaccharide manifests its functional properties by forming a molecular bridge between lipid A and O-antigen. Like lipid A, it is characterized by toxicity and exhibits weak endotoxic activity.
Lipid A is a non-standard hydroxymyristic acid. It is characterized by its linkage with a disaccharide, which anchors a LPS molecule to the bacterial outer membrane. Lipid A migrates into the blood flow if the bacterial cell is destroyed, resulting in severe toxic manifestations, including septic shock.
The product contains specific enzymes immobilized on various carriers to enhance its safety and efficacy. The joint activity of specialized sorbents and enzymes prevents the absorption of toxic products of peptide sugars enzymatic hydrolysis, of bacterial cell proteins, peptidoglycans, and other harmful metabolites of bacterial autolysis by blood. Proteolytic enzymes of the dietary supplement are not limited by the pH range of the medium, so they are active in such conditions as fungal flora dominance and digestive disorders. They also prevent sugar fermentation and protein putrefaction in the gut, thus averting intoxication and increased dysbiosis.
A possible mechanism of the product ingredients' activity in metabolic detoxification and gut biocenosis normalization is presented in Figure 1.
It is recommended to take one capsule twice a day with a meal for 30 days. The duration may be changed by a physician.
|
|
|
Figure 1. Mechanism of the product activity in metabolic detoxification and the gut biocenosis normalization |
The nutrient supply provided by the recommended dietary supplement intake is given in Table 5.
|
Table 5. Nutrient supply |
||
|
Indicator |
mg |
% of AI1 |
|
Rutin |
12 |
40 |
|
Soluble Dietary Fiber |
200 |
10 |
|
Nutritional value of 100 grams: proteins – 11 g, fats - 0.1 g, carbohydrates -0.1 g. Energy value of 100 grams: 310 kJ / 75 kcal |
||
|
AI1 – adequate intake established by Appendix 5 of “Uniform sanitary and epidemiological and hygienic requirements for products subject to sanitary-epidemiological supervision (control)” of the EurAsEC Customs Union |
||
CONCLUSION
The available pharmacological characteristics of the dietary supplement ingredients and the clinical trial results helped determine the product use as part of a comprehensive nutritional program in the following areas:
- prevention and combination treatment of candidiasis, intestinal infections, atopic dermatitis, acne, allergies, other manifestations of gastrointestinal disorders, and gut microbiota disruption;
- food and alcohol intoxication, aseptic reactions, viral and bacterial infections, and their complications, preoperative and postoperative inflammation;
- prevention of infectious complications and improvement of life quality indicators.
The developed product was tested at the enterprises of Art Life Company (the city of Tomsk) according to the requirements of national and international standards (ISO 9001, 22000, and GMP).
ACKNOWLEDGMENTS: None
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: The study was conducted according to the guidelines of the Declaration of Helsinki