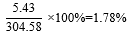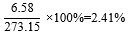Archive \ Volume.11 2020 Issue 4
Potential hair growth of crude extract from Hibiscus rosa-sinensis Linn
Laila Che Rose 1*, Nur Nadiah Syahirah Rusdi 1, Asnuzilawati Asari 1, Mohd Effendy Abd Wahid 2, Hamdan Suhaimi 1
1 Faculty of Science and Marin Environment, Universiti Malaysia Terengganu, Kuala Nerus, 21030, Terengganu, Malaysia. 2 Institute of Marine Biotechnology, Universiti Malaysia Terengganu, UMT 21030, Terengganu, Malaysia.

Abstract
The Hibiscus rosa-sinensis Linn. (HRS), the national flower of Malaysia, is famous for its use in various traditional medicine. However, its usage as an activating agent has not received much attention it deserved. Therefore, this study was directed towards the use of HRS for hair growth as a substitute for the present commercially available drug. In this work, petroleum ether was used to extract both leaves and flowers of HRS. Its potential for hair growth was evaluated in vivo. One percent crude extract of leaves and flowers of HRS in liquid paraffin was applied topically over the shaved rats of Dawley Sprague. The rats were monitored and assessed for growth of hair for 42 days. The results for the study were divided into two parts namely the hair length was determined in vivo and the histology study of the shaved area of rats. From this study, it was concluded that the leaf extract exhibited more potential for hair growth compared to the flower component. This finding is promising and serves as a potential substitute for the usage of HRS extract in hair growth treatment. In addition, being a natural product further adds its benefit as compared to synthetic chemicals which may cause harmful side effects to the consumers.
Keywords: Hibiscus, Hair growth, Histologic studies

INTRODUCTION
Medicinal agents extracted from nature has been the main source in replacing many synthetic drugs that may cause side effects [1, 2]. The most important bioactive compounds of plants are alkaloids, flavonoids, tannins and phenoliccompounds [3, 4]. There are still many ongoing studies done to discover plants that can act as medicine and use in cosmetic products. One such plant is Hibiscus rosa-sinensis Linn (HRS). It is well accepted that the whole part of HRS such as stem, roots, flower, and leaves had been used as traditional and folklore medicine [5-7]. The flowers usually used in herbal teas and food coloring and in some countries, they are eaten as salad or pickles proven. This shows that this plant is not harmful and safe to our bodies. The leaves have been used in healing processes due to their antioxidant, anti-tyrosinase, and anti-bacterial activities [8, 9]. It’s usage as traditional medicine is, however, still limited and warrants further studies. In addition, there are only a few articles on hair growth that uses HRS as an activating agent and the rest are about the anti-bacterial activity. Drugs are an option to cure hair loss, but fear of side effects cannot be ignored. An option which is using an herbal remedy to cure this disease has yet to be incorporated into the mainstream of medical care due to limited scientific evidence. Hence, it is believed that HRS provides an alternative as a herbal remedy that can be used to promote hair loss based on traditional practices. With that, in the present study, Hibiscus rosa-sinensis Linn (HRS) is used as an activating ingredient to promote hair growth. The extract from the leaves and flowers will be applied onto rats of Dawley Sprague and their hair growth will be monitored through histology study.
MATERIALS AND METHODS
Plant Material
Fresh flowers and leaves of Hibiscus rosa-sinensis Linn (HRS) were collected from Kedah and Terengganu. They were washed separately to remove dust and other pollutants and were then dried in the shade by solar drying. After the drying process, the samples were powdered using a laboratory blender and stored in a separate airtight container at room temperature. Fig. 1 and Fig. 2 show both of the dried flowers and leaves of HRS. While Fig. 3 show the flowers and leaves of HRS that had been blended using laboratory blender.

Fig. 1: Dried flowers of HRS

Fig. 2: Dried leaves of HRS

Fig. 3: Powdered leaves and flowers of HRS
Preparation of Extract
The resulting dried and powdered samples were separately subjected to Soxhlet extraction under preheated (60-80°C) petroleum ether. The extracts were collected after 24 hours. Both of the extracts were then weighed and calculated its percentage yield after solvent elimination under reduced pressure with a rotary evaporator. Then, 1g of each extract was dissolved in 100 ml of liquid paraffin to produce one percent of the active compound and the products were used for the evaluation of potential hair growth effects in vivo.
Animals
Nine rats of Sprague Dawley or known as laboratory rats, weighing 120-150g were used in this study. The cages where the rats were kept were cleaned every 4-5 days for hygiene purposes.
Preparation of anesthesia
Ketamine, xylazine, and saline mixed according to Table 1 were used as anesthesia. The anesthesia was administered to the rats before removing hair using chemical depilatory agent, before plucking hair for hair length determination and before the rats were sacrificed at intraperitoneal. The injection was administered using a 1 ml syringe with a 27 gauge needle. The volume administered to the rats per 100 g was 0.15 ml. The injection resulted in 30-60 minutes of sedation.
|
Table 1: Composition used as anesthesia |
||
|
Solutions |
Drug concentration (mg/ml) |
Volume used for cocktail (ml) |
|
Ketamine |
100 |
1.0 |
|
Xylazine |
20 |
0.5 |
|
Saline (0.9% NaCl) |
Sterile, isotonic |
8.5 |
|
Total combination |
10 |
|
Hair Removal
Chemical depilatory agent, Nair® cream was applied at 4 cm2 area of the hair on the dorsal portion of all nine rats as shown in Fig. 4. After 5-7 minutes, the cream was removed using a water-moistened gauze pad.
Fig. 4: Rat treated with hair removal cream
In Vivo Hair Growth Activity
Three of the rats were used as the control, the other three were treated with flower extract and the last three were treated with leaf extract. Approximately, 1 ml of the extracted oil was applied to the denuded area of the rats for leaf extract that were used for the experiment, and the control rats received no treatments. This treatment continued for 42 days. The hair growth pattern was observed visually and recorded accordingly. For follicular analysis, the skin biopsies were taken periodically after the application. In order to determine the hair length, 28 hairs were obtained from the affected area at random. Prior to obtaining the hairs, the rats were put under anesthesia.
Histological Studies
Skin biopsies taken from the shaved area were fixed in 10% of formalin buffer immediately after the skin was removed from the animal’s body. Water was removed from the tissue by process dehydration. Then, the samples were transferred through baths of progressively more concentrated alcohol (70% - 100%) to remove water, followed by a clearing agent, xylene in order to remove alcohol, and lastly, undergo infiltration by using molten paraffin wax to replace xylene.
After infiltration, the tissues were embedded in paraffin wax. During this process, the tissue samples were placed into molds along with the wax which was then hardened by placing on the cold plate. The hardened block containing tissue samples were then ready to be sectioned. The hardened block containing tissues are sectioned into the uniform thickness of 4-5 μm by using a microtome. The sectioned tissue then floated on warm water at 38°C in order to stretch the sectioned film and then transferred to microscopic slides. The tissues were cut into 4-5 μm for the light microscope. Finally, the sectioned tissues were stained with hematoxylin and eosin in order to study the tissue microscopically. From the section, the number of hair follicles per millimeter of the skin was determined according to Sawada et al. [10], and the percentage ratio of different cyclic phases was determined using the suitable microscope with an ocular micrometer
RESULT AND DISCUSSION
Extract of Hibiscus Rosa-sinensis Linn.
Both flowers and leaf samples of Hibiscus Rosa-sinensis Linn. (HRS) were weighed before powdered and weighed again after the extract underwent solvent elimination under reduced pressure using a rotary evaporator. The results are shown in Table 2. The color for the flower is yellow while the color for leaf extract is greenish-black as shown in Fig. 5.
|
Table 2: Results from the extraction of HRS |
||
|
Measurement |
Type of samples |
|
|
Flowers |
Leaves |
|
|
Weight of samples |
304.58 g |
273.15 g |
|
Weight of extracts |
5.43 |
6.58 g |
|
Percentage yield |
|
|

Fig. 5: Flower and leaf extract In Vivo Studies on Hair Growth
Hair growth was observed from the denuded area and the length of the hair began to increase until the end of the treatment. The hair of the rats was plucked from the random area of denuded skin and the average from 28 hairs were measured and recorded in the table below which Table 3 is the average for the rats belong to control group, Table 4 for the rats belong to leaf-extract treated group and Table 5 for the rats belong to flower-extract treated group. From the results, only rats from the leaf-extract-treated group and flower-extract-treated group show the significant growth of the hair compared to the control group. Figs. 6-8 show a comparison of rats from different groups on days 14, 28, and 42, respectively. Table 6 shows the mean ± SD that is used for statistical analysis of hair length.
|
Table 3: Average length of hair from the control group |
|||
|
Days of hair length measurement |
Control |
||
|
Rat 1 |
Rat 2 |
Rat 3 |
|
|
Day 14 |
0.19 cm |
0.20 cm |
0.19 cm |
|
Day 28 |
- |
1.00 cm |
1.08 cm |
|
Day 42 |
- |
- |
1.12 cm |
|
Table 4: Average length of hair from leaf extract-treated group |
|||
|
Days of hair length measurement |
Leaf |
||
|
Rat 1 |
Rat 2 |
Rat 3 |
|
|
Day 14 |
0.76 cm |
0.52 cm |
0.54 cm |
|
Day 28 |
- |
1.17 cm |
1.20 cm |
|
Day 42 |
- |
- |
1.20 cm |
|
Table 5: Average length of hair from flower extract-treated group |
|||
|
Days of hair length measurement |
Flowers |
||
|
Rat 1 |
Rat 2 |
Rat 3 |
|
|
Day 14 |
0.25 cm |
0.28 cm |
0.27 cm |
|
Day 28 |
- |
1.13 cm |
1.13 cm |
|
Day 42 |
- |
- |
1.20 cm |
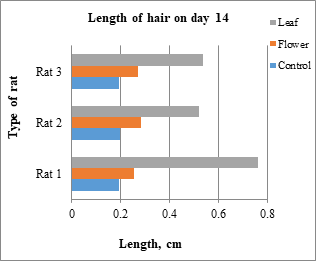
Fig.6: Type of rat vs length of hair in day 14
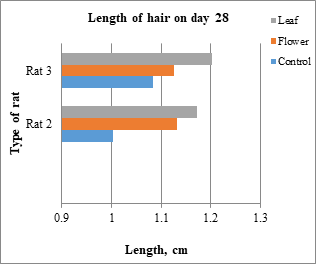
Fig. 7: Type of rat vs length of hair in day 28
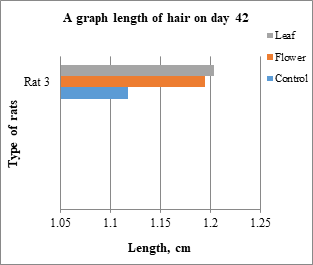
Fig. 8: Type of rat vs length of hair in day 42
|
Table 6: Student’s t-test (n=28 hairs) |
|||
|
Days of hair length measurement |
Groups |
||
|
Control |
Leaf |
Flower |
|
|
Day 14 |
0.19 ± 0.05 |
0.60 ± 0.14 |
0.27 ± 0.06 |
|
Day 28 |
1.04 ±0.08 |
1.19 ± 0.11 |
1.13 ± 0.10 |
|
Day 42 |
1.12 ± 0.08 |
1.20 ± 0.09 |
1.20 ± 0.09 |
Morphology Observation
Fig. 9 to Fig. 11 show the comparison pattern of baldness between control, leaf, and flower groups respectively on days 14, 28, and 42. For rats from the control group on day 14, the hairs started to grow at the shaved area. However, the hair growth pattern is not uniform because some shaved areas are still not covered with hair. The hairs started to cover half of the shaved area on day 28, but there are areas with less hair and bald. On the last day, which is on day 42 the hair did not completely cover the whole shaved area, plus the thickness for the hair at the shaved area was not uniform. For rats from the flower-extract group, the hairs start to grow on half of the shaved area. On day 28, the hairs almost cover all the shaved area but there are areas with no hair or bald. On day 42, the hairs cover all the shaved area but with non-uniform thickness. Opposite to that, the rat treated with leaf extract gave positive results at day 14. The whole shaved area which is an area of treatment had completely covered with hairs without a sign of baldness. On day 28, the hairs at the shaved area grow in a uniform thickness, and on day 42, the hairs completely grow.
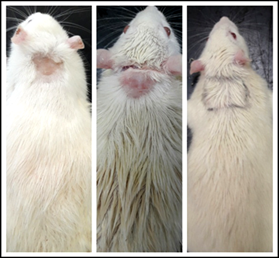
Fig. 9: Rat from control group on days 14, 28 and 42
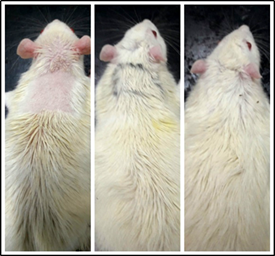
Fig. 10: Rat from leaf extract group on days 14, 28 and 42
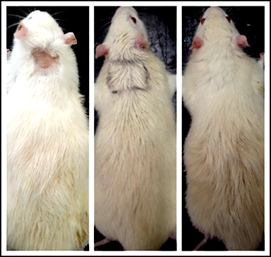
Fig. 11: Rat from flower extract group on days 14, 28 and 42
It was observed that the hair growth initiated from the shaved area in the second week in all groups including the control group and the whole denuded area was covered by the end of the course. The difference was the thickness of the hair growth in all groups. The observation for the treated groups which are flower extract group and leaf extract group showed a significant result. This might be due to the gentle rubbing on the shaved skin while applying the extract since the act of the rubbing may enhance the blood circulation in the local area and thus exert some effect on hair growth. Plus, it gave a relaxing feeling since stress is one of the factors of hair loss [11]. Another reason is because of the effect of petroleum ether extract of HRS used in both leaf and flower extract. There are many studies that used petroleum ether in the extraction and gave a positive result on hair growth. For example, petroleum ether root extract of Glycyrrhiza Glabra or liquorice in rats showed a maximum number of anagenic hair and higher follicle density [12] and petroleum ether extract of Cuscuta reflexa (dodder) also showed potential as hair growth-promoting agent in androgenetic alopecia [13].
Histological Studies
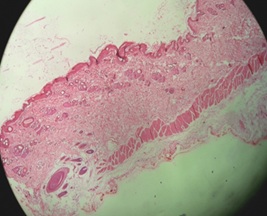
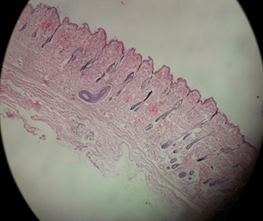
Fig. 12 (a) and (b) show the image of the skin at the shaved area for the control group on days 28 and 42 respectively. The skins of the rats were taken on days 28 and 42 for histological studies. Haematoxylin and eosin staining was used as stains in this study. On day 28, there were only hair follicles without hair shaft. On day 42, the skin started to grow where the hair follicle started to produce hair shaft and enter the surface of the skin.
 |
|
(a) |
 |
|
(b) |
Fig. 12: Control group skin on the day (a) 28 and (b) 42
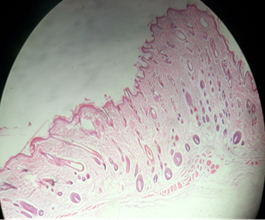
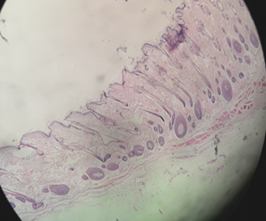
Fig. 13 (a) and (b) show the image of the skin at the area of treatment for flower extract group on days 28 and 42 respectively. On day 28, there was abundance of hair follicles compared to control group skin on day 28 (Figure 4.8). On day 42, hair bulbs started to thicken and hair shaft started to grow.
 |
|
(a) |
 |
|
(b) |
Fig. 13: Flower extract group skin on the day (a) 28 and (42)
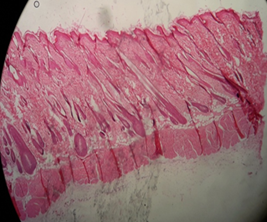
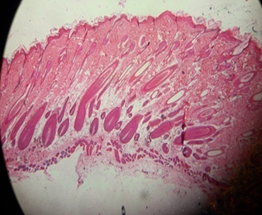
Both Fig. 14 (a) and (b) show equivalent images but for the leave extract. On day 28, the skin already had hair shaft which is the hair growth is faster than both the control group and flower extract group. On day 42, the hair bulbs started to thicken.
 |
|
(a) |
 |
|
(b) |
Fig. 14: Leaf extract group on the day (a) 28 and (b) 42
For this study, there should be hair density counting result and hair follicles counting result. However, the lack of time cause the other test cannot be run. A marked difference in the hair bulbs and hair shaft for all the rats can be seen from all the figures shown. Plus, the hair for both control and flower extract group was looking sparse compared to leaf extract group. Overall, there are no signs of infection an allergic reaction on the area of treatment for treated group rats.
CONCLUSION
In conclusion, the in vivo and histology study on rats had showed that the leaf extract has impact on hair follicle and thus may improve hair growth. Petroleum ether is suitable to be used as a solvent to extract the component in both flowers and leaves of Hibiscus rosa-sinensis Linn. (HRS) as there are no signs of infection an allergic reaction on the area of treatment. Moreover, it gives positive result on hair growth. The leaves and flower extract-treated groups also produce a significant effect with respect to the control. But, the leaves extract-treated group showed more positive result compared to the flower extract-treated group.
Declaration of interest
This work is supported by Fundamental Research Grant from Ministry of Higher Education, Malaysia (Grant No. 59364) and is gratefully acknowledged.
REFERENCES
- Sargia B, Singh B, Gupta N, Gahlot L K, Gulati T, Hasija Y. MED-PDB: An online database of medicinal plants. J. Adv. Pharm. Edu. Res. 2018; 7(4): 204-207.
- Kianitalaei A, Feyzabadi Z, Hamedi S, Qaraaty M. Althaea Officinalis in Traditional Medicine and modern phytotherapy. J. Adv. Pharm. Edu. Res. 2019; 9(S2): 154-161.
- Ahmad M S, Shawky A, Ghobashy M O, Felifel R H A. Effect of Some medicinal plants on life cycle of Citrus Brown Mites (Eutetranychus orientalis). Int. J. Pharm. Res. Allied Sci. 2017; 6(1): 53-58.
- Benzineb E, Kambouche N, Hamiani A, Bellahouel S, Zitouni H, Toumi H. Phenolics Compounds and Biological Activity of Leaves of Anabasis Articulata, an Algerian Medicinal Plant. Int.J. Pharm. Res. Allied Sci. 2019; 8(4): 1-5.
- Adhirajan, N., Kumar, T.R., Shanmugansundaram, N., Babu, M. In vivo and in vitro evoluation of hair growth potential of Hibiscus rosa-sinensis Linn. Journal of Ethnopharmacology 2003; 88: 235-239.
- Anita Gnana Kumari, A.V., Palavesam, A., Anbu Jeba Sunilson, J., Anandarajagopal, K., Vignesh M., Parkavi, J. Preliminary phytochemical and antiulcer studies of Hibiscus rosa sinensis Linn. root extracts. International Journal Green Pharmacy 2010; 4:41-43.
- [Nade, V.S., Kanhere, S.V., Kawale, L.A., Yadav, A.V. Cognitive enhancing and antioxidant activity of ethyl acetate soluble fraction of the methanol extract of Hibiscus rosa sinensis in scopolamine-induced amnesia. Indian Journal of Pharmacology 2011; 43(2):137–142.
- Nivsarkar, M., Patel, M., Padh, H., Bapu, C., Shrivastava, N. Blastocyst implantation failure in mice due to “nonreceptive endometrium”: endometrial alterations by Hibiscus rosa-sinensis leaf extract. Contraception 2005; 71(3): 227-230.
- Shen, H.M., Chen, C., Jiang, J.Y., Zheng, Y.L., Cai, W.F., Wang, B., Ling, Z., Tang, L., Wang, Y.H., Shi, G.G. The N-butyl alcohol extract from Hibiscus rosa-sinensis L. flowers enhances healing potential on rat excisional wounds. Journal of Ethnopharmacology 2017; 198: 291-301.
- Sawada M., Terada N., Tangiguchi H., Tateishi R., Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest, 1987; 56: 684-686
- Takeo Mitsui. New Cosmetics Science. Amsterdam: Elsevier Science, 1997.
- Upadhyay, S., Ghosh, A.K., Singh, V. Hair Growth Promotant Activity of Petroleum Ether Root Extract of Glycyrrhiza Glabra L (Fabaceae) in Female Rats. Tropical Journal of Pharmaceutical Research 2012; 11(5): 753-758.
- Patel, S., Nag, M.K., Sharma, V., Chauhan, N.S., Dixit, V.K. A comparative in vivo and in vitro evaluation of hair growth potential of extracts and an isolate from petroleum ether extract of Cuscuta reflexa Roxb. Beni-Suef University Journal of Basic and Applied Sciences 2014; 3(3): 165-171.