Archive \ Volume.14 2023 Issue 4
The Expressions of Sodium Chloride Cotransporter (NCC mRNAs) in the Kidney Hypertensive Rats
Nimer Alsabeelah1*
1Department of Pharmacy Practice, College of Pharmacy, University of Hafr Al Batin, Hafr Al Batin, 39524, Saudi Arabia.
Abstract
The present study hypothesized that chronic administration of candesartan will not only improve the sensitivity of alpha-adrenergic receptors to adrenergic agonists but also modulate the expression of angiotensin receptors (AT1a) and sodium chloride cotransporter (NCC) mRNAs. Animals were divided into four groups WKY control; WKY received candesartan (WKY-CST, 10 mg/kg), SHR control, and SHR received candesartan (SHR-CST). Plasma, urine samples, and mean arterial pressure (MAP) were taken on days 0, 21, and 28. Acute studies determined the renal vasoconstrictor actions of Ang II, noradrenaline (NA), phenylephrine (PE), and methoxamine (ME). The overall mean drops in renal cortical blood perfusion (RCBP) to NA, PE, ME, and Ang II were significantly increased in WKY-CST and SHR-CST when compared to the respective control. Expression of AT1a mRNA in the kidney of WKY-CST and SHR-CST were increased 11 folds and 8 folds respectively when compared to internal control (β-actin). Expression of NCC MRNA in the kidney of WKY-CST and SHR-CST were 10 folds and 6 folds respectively when compared to internal control. Candesartan restores the functional responsiveness of alpha-adrenergic receptors in normal and spontaneously hypertensive rats by alleviating mean arterial pressure, enhancing renal cortical blood perfusion, and increasing the functional capabilities of the kidney by modulating renal tubular and glomerular functions. Secondly, chronic administrations of candesartan in SHR regulated the expression of AT1a mRNA and NCC mRNA in SHR rat kidneys when compared to SHR control rats.
Keywords: Hypertensive nephropathy, Candesartan, Sodium chloride cotransporter, Angiotensin II, Alpha-adrenergic receptors
INTRODUCTION
Hypertension is a common disorder that affects a large population and Europe is considered a high-risk region for hypertension [1]. Hypertension is mostly associated with some risk factors which are responsible for the onset of hypertension [2, 3]. Among those factors are nutrient intake, obesity, physical activity, alcohol intake, environmental toxins, psychosocial stressors, and genetic susceptibility [4]. Angiotensin II (Ang II), the main effector of RAS, is a potent vasoconstrictor hormone that controls blood pressure and volume in the cardiovascular system [5]. Angiotensin II (Ang II), the main effector of RAS, is a potent vasoconstrictor hormone that controls blood pressure and volume in the cardiovascular system [5].
The angiotensin II type 1 (AT1) receptor is thought to mediate the major cardiovascular effects of Ang II [6, 7]. The Ang II receptor blockers (ARBs) bind to the AT1 receptor with high affinity and were found to be greatly selective for the AT1 receptor compared to the AT2 receptor [8, 9]. Two subtypes of AT1 receptor (AT1A and ATIB) have been discovered with highly homologous sequences and similar binding and functional characteristics [10, 11]. The AT1A receptor is predominantly expressed in the kidney [12] and is the murine homologue to the single human AT1 receptor [13].
There is evidence indicating that the kidneys, brain, heart, and vasculature contain all components of RAS mRNA, and are thus capable of producing Ang II locally [5, 14]. Many studies reported the mRNA expression of AT1A during treatment with AT1 receptor antagonists in hypertensive rats [15]. Brooks et al. showed that knockout mice with AT1A had reduced renal abundance of sodium chloride cotransporter (NCC) [16]. The sodium-chloride (Na-Cl) cotransporter NCC is functionally expressed on apical membranes of the renal distal convoluted tubule [17, 18]. It is considered a major protein responsible for electrolyte homeostasis and the reabsorption of approximately 10% of sodium in the kidney [19]. Another study found that chronic RAS blockade decreased apical membrane-associated NCC in spontaneously hypertensive rats (SHR) [20]. Many studies in the 1990s have pointed to NCC as a potential candidate in the development of hypertension in genetic models [21]. However, in 2001, Moreno and colleagues proposed that a change in mRNA levels of NCC is not associated with the development of hypertension in spontaneously hypertensive rats, in rats with renovascular hypertension, nor rats with hypertension induced by nitric oxide synthesis inhibition [22]. Beutler and colleagues found no effect of candesartan treatment on NCC abundance in low NaCl-fed rats after 2 days of treatment [23]. Thus, the regulation of renal NCC levels by the AT1A receptor is not well understood while the impact of candesartan on the expression levels of AT1a and NCC in the kidney remained unclear in normal and spontaneously hypertensive rats [24, 25].
It has been observed that the onset of hypertension ameliorates the functional capabilities of the kidney but also reduces the responsiveness of the alpha-adrenergic receptors [26]. Responsiveness of a1B adrenergic receptors has been found to decline in fructose-fed rats where the Ang II level was elevated [27]. Responsiveness of a adrenergic receptors has declined in adrenergic and angiotensin II-induced vasoconstriction [28]. Responsiveness of adrenergic receptors has been reported to reduce left ventricular hypertrophy where Angiotensin II was elevated [29, 30]. Limited studies have paid attention to restoring kidney functions by increasing the responsiveness of a adrenergic receptors in left ventricular hypertrophy (LVH) [29, 31] and fructose-fed rats by the administration of candesartan [32]. Restoration of responsiveness of a adrenergic receptors in hypertension remained folded [33, 34].
None of the studies has observed the impact of candesartan on the responsiveness of responsiveness of a adrenergic receptors to its agonists in normal and spontaneously hypertensive rats. Secondly, none of the studies measured the impact of candesartan on the expression of sodium chloride cotransporter channel (NCC) along with ATI1a mRNA expression in the kidney [35, 36]. The present study set with the objective of whether chronic administrations of candesartan restore the functional responsiveness of alpha-adrenergic receptors in normal and spontaneously hypertensive rats. Secondly, the present study set out to investigate the impact of chronic administration of candesartan on the expression of AT1a MRNA and NCC mRNA in normal and spontaneously hypertensive rats [37].
MATERIALS AND METHODS
Experimental Groups
Male Wistar Kyoto (WKY) and Spontaneously Hypertensive rats (SHR) weighing 180-220 g (at the start of the experiment) were procured. from the Animals Breeding House, Department of Pharmaceutical Sciences, Government College University Faisalabad, Pakistan. The animals were housed in a well-ventilated transit room. at the Department of Pharmaceutical Sciences, Animal Breeding House, Government College University Faisalabad, Pakistan. All animals were given a commercial brand for rat chow and water ad libitum. All in vivo study was performed after the approval of the Institutional Review Board for Animal Studies (Study No 19680/IRB No 680), Government College University Faisalabad, Pakistan, and were carried out per the provided guidelines. Animals were divided into 4 groups (n=7); (1) WKY-control, WKY treated with candesartan (WKY-CST), SHR control and SHR treated with candesartan (SHR-CST) while similar groups were recruited for molecular expression study for AT1a and NCC mRNAs (n=3). All animals were kept in a controlled environment of temperature, light and dark cycle, and continuous supply of food and water.
Treatment with Candesartan
Candesartan (cilexetil AstraZeneca AB, Sodertalje, Sweden) was given orally daily for 7 consecutive days before acute study at a dose of 10 mg/kg as reported [38]. The stock solution for candesartan was prepared at 10 mg/ml. Candesartan was dissolved in distilled water and freshly prepared every day.
Measurement of Non-Invasive Blood Pressure (NIBP) in Conscious State
Noninvasive blood pressure measurement was done by using the tail-cuff method CODATM (Kent Scientific Corporation, Torrington, Connecticut, USA) on days 0 and 21 of the study period as reported [29]. The setting of the apparatus consists of an occlusion cuff and a VPR sensor principle is similar to a sphygmomanometer. Both O-cuff and VPR were placed on the tail and the system is attached to the data acquisition system which is attached to a computer having inbuilt software version 3.1 (Kent Scientific Corporation).
Acute Experiment for Systemic Hemodynamic and Renal Cortical Blood Perfusion
Surgical Procedure
All the rats were put on fasting for 12 hours before undergoing anesthesia and acute surgical experiments. Animals were anesthetized with a 60mg/kg dose of pentobarbital sodium (Dorminal 20% - Alfasan, Woerden, Holland) via the intra-peritoneal route. A maintenance dose of 15mg/kg (diluted) will be administered through the jugular vein if required during the experiment. The onset of anesthesia will be confirmed by observing the reflexes like mechanical pressing of the tail movement of the eye and blinking of the eye. After the confirmation of the onset of the anesthesia, the animal was put on the acute experiment table in the dorsal position. An acute experiment was done as reported earlier [39-41]. To avoid the fur during the acute experiment, the abdominal area was shaved with the hair clipper (WAHL Clipper Corporation, USA) which will ease the peritoneum of the animal so that the kidney can be exposed. Immediately, after the onset of anesthesia, a tracheotomy was done by using an endotracheal tube (PP 240, Portex Ltd. Kent, UK). The left jugular vein was cannulated by using PP 50 (Portex Ltd. Kent, UK) to facilitate the provision of maintenance doses of anesthesia, saline, and different drugs if required. The right carotid artery was cannulated by using same-sized PP 50 tubing (Portex Ltd. Kent, UK) which was connected to a heparin saline-filled pressure transducer (P23 ID Gould, Statham Instruments, UK) that was linked to a data acquisition system (PowerLab®, ADInstruments, Sydney, Australia) through a Quad Amp (ADInstruments, Australia) for the measurement of MAP.
After the completion of surgery at the neck area, a midline longitudinal abdominal incision was given and to avoid bleeding electrical cautery (Rimmer Brothers, UK) was used to open the abdomen up to the region of the rib cage. All the organs of the abdomen were moved to the right side of the rat with cotton which was soaked with saline to avoid dehydration and evaporation. The Iliac artery was isolated and cannulated with PP 50 tubing (Portex Ltd. Kent, UK) and the cannula was pushed up to the point of bifurcation of the aorta toward the iliac artery. The pressure transducer (P23 ID Gould, Statham Instruments, UK) was then linked to the iliac cannula using the same connection method as the carotid artery cannulation. To shield the kidneys from the heat from the lamp, they were maintained submerged in salt water. The laser Doppler flow probe (OxyFlow, ADInstruments, Australia) was used to evaluate the renal cortical blood perfusion in the left kidney of the rats by positioning it on the kidney's cortex. After placing the laser probe, the bladder was cannulated with PP50 tubing (Portex Ltd. Kent, UK) to ease the process of urination during the study. The animal was allowed to stabilize for a minimum of 45 minutes and then baseline readings of arterial blood pressure, heart rate, and renal cortical blood pressure were measured.
Acute Vasoconstrictor Response Study
After taking the baseline reading of systemic hemodynamics, the iliac cannula was pushed further up to the point of the renal artery in such a way that the tip of this beveled cannulation was on the face of the renal artery. This technique was done to facilitate the provision of different agonists and antagonists directly to the kidney and avoid their effect on systemic circulation as reported [32, 40, 42]. This iliac cannula was connected to a pressure transducer on one side while one opening was for the provision of acute doses of agonists like noradrenaline (NA), phenylephrine (PE), methoxamine (ME), and angiotensin II (Ang II) [32, 40, 43, 44]. The end of this cannula was connected to a perfusion pump speed which was 6ml/hr. preparation of doses and administration of the doses was followed as reported earlier [39, 40, 44, 45]. NA, PE ME, and ang II were administered intrarenal first in ascending order and then in descending order of each dose of agonist to get an average of one dose. NA doses were 25, 50,100, and 200 ng, PE were 0.25, 0.5,1, and 2 µg, doses of ME were 1, 2, 3, and 4 µg while the doses of Ang II were 2.5ηg,5.0ηg, 10ηg, and 20ηg. These doses were prepared in normal saline freshly every day and stored at 4 ºC [46, 47]. A period of 12 min was allowed between each dose administration to ensure washout [48, 49].
Preparation of Vasoactive Agents
Dilution of stock solutions was undertaken in normal saline immediately before use. Noradrenaline, phenylephrine, methoxamine, and angiotensin II were diluted fresh daily from frozen stock containing 10 mg/ml, 100 mg/ml, 2 mg/ml, and 10 mg/ml, respectively.
Molecular Study Using Quantitative Real-Time PCR (qPCR)
Relative Quantification
Quantitative real-time PCR (qPCR) was used for the relative quantification of AT1A and NCC mRNAs in the kidneys of both groups WKY and SHR. Gene expression is analyzed by using the Comparative CT Method (ΔΔ CT Method). The method of mRNA expression was followed as reported [31].
RESULTS AND DISCUSSION
Renal Hemodynamic Data of WKY Control, WKY-CST, SHR Control, and SHR-CST on Days 0, 21, and 28.
Fractional excretion of sodium (FENa) of WKY control, WKY-CST, SHR control, and SHR-CST was calculated on days 0, 21, and 28. FENa (%) of SHR control was significantly reduced (p<0.05) than the FENa (%) of WKY control on days 0, 21, and 28. After the treatment of WKY control and SHR control with candesartan, FENa (%) of SHR-CST was significantly increased (P<0.05) when compared to SHR control but still significantly less (p<0.05) than WKY control on day 28 as shown in Table 1.
|
Table 1. Renal hemodynamic parameters obtained on days 0, 21, and 28 in WKY control, WKY-CST, SHR control, and SHR-CST. |
|||||
|
Parameters |
Day |
WKY control |
WKY- CST |
SHR control |
SHR-CST |
|
FE-Na (%) |
Day 0 |
0.6+0 |
0.6+0 |
0. 34+0* |
0. 35+0* |
|
Day 21 |
0.6+0 |
0.6+0 |
0. 3+0.0* |
0. 3+0* |
|
|
Day 28 |
0.6+0 |
0.6+0 |
0. 3+0* |
0.5+0*# |
|
|
Cr. Cl |
Day 0 |
0.6+0 |
0.5+0 |
0.23+0 |
0.22+* |
|
Day 21 |
0.5+0 |
0.5+0 |
0.22+0 |
0.2+0* |
|
|
Day 28 |
0.5+0 |
0.5+0 |
0.2+0 |
0.4+0*# |
|
|
KI (%) |
Day 28 |
0.3+0 |
0.3+0 |
0.4+0* |
0.30+0# |
The values are presented as mean ± S.E.M. (n = 6). Statistical analysis was performed by one-way ANOVA followed by Bonferroni post hoc test. Values with P < 0.05 were considered statistically significant. *P<0.05 vs. WKY control on same day while # P<0.05 vs. SHR control on same day.
Creatinine clearance (Cr.Cl.) of WKY control, WKY-CST, SHR control, and SHR-CST was calculated on days 0, 21, and 28. Cr.Cl (ml/min/100 g b.w.) of SHR control was significantly reduced (p<0.05) than the Cr.Cl (ml/min/100 g b.w.) of WKY control on days 0, 21 and 28. After the treatment of WKY control and SHR control with candesartan, Cr.Cl (ml/min/100 g b.w.) of SHR-CST was significantly increased (P<0.05) when compared to SHR control but still significantly less (p<0.05) than WKY control on day 28 as shown in Table 1.
The kidney index was measured on day 28 at the end of the acute experiment and it was observed that the kidney index of the SHR control was significantly greater (p<0.05) than the WKY control. Treatment with candesartan significantly reduced (p<0.05) the kidney index in SHR-CST when compared to SHR control but comparable to WKY control on day 28 as shown in Table 1.
Mean Arterial Pressure of WKY Control, WKY-CST, SHR Control, and SHR-CST on Days 0, 21, and 28
Mean arterial pressure (MAP) of WKY control, WKY-CST, SHR control, and SHR- CST on day 0 was measured as 97, 100, 142, and 140 mmHg respectively which clearly indicates that the MAP of SHR control and SHR-CST was significantly higher (p<0.05) to WKY control, WKY-CST. Similarly, MAP of WKY control, WKY-CST, SHR control, and SHR-CST on day 21 was also measured as 102,100, 135, and 135 mmHg respectively as shown in Figure 1. After 1 week of treatment with candesartan on day 28, MAP of WKY-CST and SHR-CST was significantly reduced (p<0.05) from 100 to 75 mmHg and 135 to 102 mmHg respectively as shown in Figure 1.
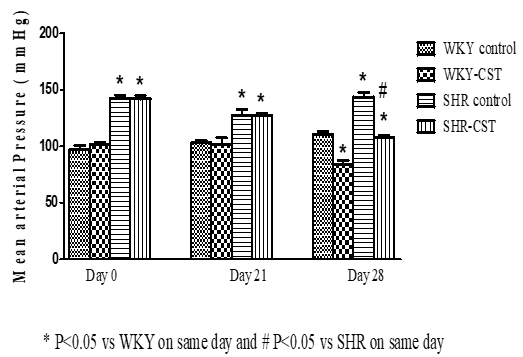 |
|
Figure 1. Mean arterial pressure of WKY control and WKY-CST rats at days 0, 21, and 28. |
Baseline Renal Cortical Blood Perfusion (RCBP) of WKY Control, WKY-CST, SHR Control, and SHR-CST on Day 28
RCBP was measured on day 28 after 1 week of treatment with candesartan. RCBP values in WKY control, WKY-CST, SHR control, and SHR-CST were measured as 170, 172, 135, and 175 % RCBP respectively indicating that significantly increased (p<0.05) % RCBP in SHR-CST when compared to SHR control and comparable to WKY group as shown in Figure 2.
 |
|
Figure 2. Baseline renal cortical blood perfusion (RCBP) on day 28 in WKY control and WKY-CST rats. |
Relative Quantification of Kidney AT1a (mRNA Expression) in WKY Control, WKY-CST, SHR Control, and SHR-CST at the End of the Treatment Period
There was a significant increase (P<0.05) expression of AT1a in the kidney of SHR control, SHR-CST about 11 and 8 folds when compared to the expression of the same mRNA in the kidney of the WKY Control group as shown in Figure 3. Expression of AT1a in the kidney of SHR-CST was significantly less (P<0.05) than SHR control.
Relative Quantification of Kidney NCC (mRNA Expression) in WKY Control, WKY-CST, SHR Control, and SHR-CST at the End of the Treatment Period
There was a significant increase (P<0.05) expression of NCC in the kidney of SHR control, SHR-CST about 9 and 7 folds when compared to the expression of the same mRNA in the kidney of the WKY control group as shown in Figure 4. However, the expression of NCC in the kidney of SHR-CST was significantly less (all P<0.05) than that in SHR control.
 |
|
Figure 3. Relative quantification of kidney AT1a (mRNA expression) in control WKY control, WKY-CST, control SHR, and SHR-CST at the end of the treatment period |
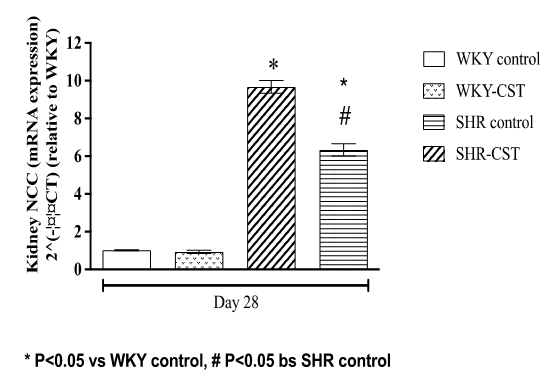 |
|
Figure 4. Relative quantification of kidney NCC (mRNA expression) in control WKY control, WKY-CST, control SHR, and SHR-CST at the end of the treatment period. |
Mean fold changes (normalized to β-actin and relative to WKY) were calculated by the 2-ΔΔCT method. The values are presented as mean ± S.E.M. (n = 3, triplicate samples from each rat). Statistical analysis was performed by one-way ANOVA followed by Bonferroni post hoc test. Values with P < 0.05 were considered statistically significant.
Overall Mean Percentage Drop in the Renal Cortical Blood Perfusion in Response to NA, PE, ME, and Ang II in WKY control, WKY-CST, SHR Control, and SHR-CST
Noradrenaline
Exogenous administration of noradrenaline (NA) close to the renal artery in WKY control, WKY-CST, SHR control, and SHR-CST resulted in renal vasoconstriction at different doses of NA (25ɳg, 50ɳg, 100ɳg and 200ɳg) in a dose-dependent manner as shown in Figure 5a. Minimum responses were observed at lower doses which increased with an increase in dose until the highest renal vasoconstriction was observed at the highest dose 200ɳg as shown in Figure 3. Overall renal vasoconstriction observed in WKY Control is 35 % which is significantly greater (P<0.05) than the responses produced by NA in SHR as shown in Figure 5 and SF.1-A. However, renal vasoconstriction responses produced by NA in SHR-CST were significantly greater (P<0.05) than the responses produced by NA in SHR as shown in Figure 5 and SF.1-A.
Phenylephrine
Exogenous administration of phenylephrine (PE) close to the renal artery in WKY control, WKY-CST, SHR control, and SHR-CST resulted in renal vasoconstriction at different doses of PE (0.25μg, 0.50μg, 1.0μg, and 2.0μg) in a dose-dependent manner as shown in Figure 5b and SF.1-B. The minimum response was observed at lower doses which increased with an increase in dose until the highest renal vasoconstriction was observed at the highest dose 2.0 μg as shown in SF.1-B. Overall renal vasoconstriction observed in SHR is 25 % which was significantly less (P<0.05) than the responses produced by PE in WKY. However, renal vasoconstriction responses produced by PE in SHR-CST were significantly greater (all P<0.05) than the responses produced by PE in SHR control as shown in Figure 5b and SF.1-B.
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
*P<0.05 vs. WKY; # P<0.05 vs. SHR |
|
Figure 5. a-d) Overall mean percentage drop in the renal cortical blood perfusion in response to NA, PE, ME, and Ang II in WKY control, WKY-CST, SHR control, and SHR-CST. |
Methoxamine
Exogenous administration of methoxamine (ME) close to the renal artery in WKY control, WKY-CST, SHR control, and SHR-CST resulted in renal vasoconstriction at different doses of ME (0.5μg, 1μg, 2μg, and 4μg) in a dose-dependent manner as shown in Figure 5c. Minimum responses were observed at lower doses which increased with the increase in dose until the highest renal vasoconstriction response was observed at the highest dose of 4μg as shown in Figure 5c and SF.1-C. Overall renal vasoconstriction observed in SHR was 18 % which is significantly less (all P<0.05) than the responses produced by ME in WKY control. However, renal vasoconstriction responses produced by ME in SHR-CST were significantly greater (P<0.05) than the responses produced by ME in SHR control as shown in Figure 5c and SF.1-C.
Angiotensin II
Exogenous administration of angiotensin II (Ang II) close to the renal artery in WKY control, WKY-CST, SHR control, and SHR-CST resulted in renal vasoconstriction at different doses of Ang II (25ng, 50ng, 100ng, and 200ng) in a dose-dependent manner as shown in Figure 5d and SF.1-D. Minimum responses were observed at lower doses which increased with an increase in dose until the highest renal vasoconstriction response was observed at the highest dose 200ng. Overall renal vasoconstriction observed in SHR control is 21 % which is significantly less (p<0.05) than the response produced by Ang II in WKY control. Exogenous administration of Ang II in SHR-CST resulted in significantly blunted (P<0.05) renal vasoconstriction responses to 7 % which is significantly less (all P<0.05) than the responses produced by Ang II in WKY control and SHR control as shown in Figure 5d and SF.1-D.
The present study set out with the objective to determine whether chronic administrations of candesartan restore the functional responsiveness of alpha-adrenergic receptors in normal and spontaneously hypertensive rats. Secondly, the present study set out to investigate the impact of chronic administration of candesartan on the expression of AT1a MRNA and NCC MRNA in the kidneys of normal and spontaneously hypertensive rats. The current study explored that treatment with candesartan increases the responsiveness of a-adrenergic receptors of the kidney to its adrenergic agonist in spontaneously hypertensive rats. Secondly, chronic administrations of candesartan in SHR downregulated expression of AT1a mRNA and NCC mRNA in rat kidneys where the blockade of AT1a mRNA by candesartan influences NCC mRNA expression and functions but untrue vice versa as downregulated NCC mRNA did not enhance the responsiveness of AT1a mRNA to its agonist as it does with adrenergic receptors to its adrenergic agonists.
The present study has shown significant differences in renal function parameters (FENa and creatinine clearance) in SHR control which justifies the notion of hypertension-induced renal damage or end-stage renal disease (ESRD) [50, 51]. The present study measured fractional excretion of sodium (FENa) and creatinine clearance (Cr.Cl) as a marker of kidney function as reported [52] to assess the impact of hypertension on kidney and treatment modulation in renal functions of hypertensive rats. Our study has shown the restoration of kidney parameters after treatment with candesartan in SHR (SHR-CST) by improving the FENa (%) and creatine clearance when compared to the SHR control group as shown in Table 1. Improved FENa in the SHR-CST group indicates that tubular functions of the nephron were restored by treatment with candesartan which supports the notion that ARBs protect the kidney [53]. Renal tubules are responsible for the handling of all electrolytes including sodium [54, 55]. ARB's protection of the kidney may be multifactorial but one of the possible reasons is by restoration of renal tubular function. Along with improved fractional excretion of sodium, candesartan treatment to SHR groups has improved creatinine clearance as shown in Table 1 indicates that candesartan also has an impact on the functional capacity of glomerulus as creatinine clearance is considered a relative marker of glomerulus filtration rate (GFR) [56, 57]. It can be speculated that ARBs like candesartan provide protection to the kidney in spontaneously hypertensive rats by improving the functional capabilities of renal tubules (proximal and distal convoluted tubules) and glomerulus (by increasing glomerular filtration rate).
Another possible reason for renal protection by candesartan along with improved renal tubules and glomerulus capabilities is the ability of candesartan to lower mean arterial pressure as shown in Figure 1. Present study showed that treatment of SHR with candesartan has reduced MAP significantly when compared to untreated SHR and WKY control at day 28 as shown in Figure 1. this finding of lowering MAP by candesartan in SHR is a universal fact and in cohesion with many studies [58, 59]. Levels of Ang II are elevated in SHR as reported [60] which is usually a reason for elevated MAP and it clearly makes sense that the administration of candesartan has reduced the elevated MAP in SHR. At the same time, studies have reported that elevated Ang II has resulted in reduced renal cortical blood perfusion by suppressing NO/cGMP signaling and endothelial dysfunction in this animal model [61]. Our findings are in cohesion with the findings that elevated Ang II has reduced the renal cortical blood perfusion while blockade of Ang II receptors (AT1a) in the kidney might have offset the vasoconstriction produced by Ang II-AT1a axis in the kidney and resulted in elevation of RCBP. Another possibility is the recovery of the NO/cGMP signaling pathway in the kidney which was suppressed due to Ang II [61]. It has been reported that the upregulation of eNOSNO/cGMP pathways and antagonizing the action of Ang II in the kidney resulted in enhanced RCBP [29, 31, 62].
Enhanced RCBP in the SHR-CST treatment group indicates that the AngII-AT1a axis is modulated when SHR was treated with candesartan (SHR-CST. The present study went one step down to explore the expression of AT1a mRNA in the kidney and found significant upregulation of AT1a mRNA expression in the kidney of SHR as shown in Figure 3 which was the reason for reduced RCBP but at the same time elevation of MAP in SHR group as shown in Figures 1 and 2. Treatment of SHR with candesartan (SHR-CST) has significantly downregulated the mRNA expression in the kidney as shown in Figure 3 which also augmented the RCBP and lowered MAP. This downregulation of AT1a mRNA in the SHR-CST group is in agreement with the study which demonstrates that candesartan downregulated the AT1a mRNA expression in the brain [63] and in the kidney [64]. By connecting the dot between NCC mRNA and AT1a mRNA both are responsible for the onset of hypertension as reported that NCC is a potential candidate in the development of hypertension in genetic models [21]. The present study measured the expression of NCC mRNA in the kidney of SHR control which was 10-fold elevated when compared to WKY control as shown in Figure 4. treatment with candesartan (SHR-CST) has downregulated the expression of NCC mRNA 4 folds when compared to SHR control rats as shown in Figure 4. This indicates that candesartan treatment in SHR downregulated the expression of NCC mRNA and ATI1a mRNA in the kidney which not only involved in lowering of MAP but also enhancement of RCBP as shown in Figures 1 and 2 respectively.
The present study observed the decreased responsiveness of alpha-adrenergic receptors of the kidney to its adrenergic agonists like noradrenaline, phenylephrine, methoxamine, and angiotensin II in SHR control when compared to WKY control as shown in graded doses response curve (SF.1-A-D) and overall mean percentage drop in RCBP Figures 5a-5d. These findings are in agreement with previous studies that reported similar findings in SHR [38]. Our study is also in agreement with a previously reported study [38] that candesartan treatment has enhanced the responsiveness of alpha-adrenergic receptors of the kidney to its adrenergic agonists. The current study explored that this reduced responsiveness of alpha-adrenergic receptors of the kidney to its adrenergic agonists in SHR control was due to the upregulation of NCC mRNA and AT1a mRNA in the kidney. This might be attributed to enhanced sympathetic tone may release of renin and consequently enhancement of the AT1a-Ang II axis which leads to reduced responsiveness of adrenergic receptors as reported [65]. Treatment with candesartan in WKY control, WKY-CST, SHR control, and SHR-CST has significantly downregulated (P<0.05) the expression of NCC mRNA and AT1a mRNA in the kidney but also augmented the responsiveness of alpha-adrenergic receptors of the kidney to its adrenergic agonists in SHR-CST as shown in Figures 5a-5d and SF.1 (A-D). Interestingly, candesartan treatment enhanced the sympathetic pathway by increasing the responsiveness of alpha-adrenergic receptors of kidney to its adrenergic agonists in SHR-CST groups but responsiveness of AT1a mRNA to its agonist Ang II remained suppressed indicates that AT1a is dominant receptors in the kidney and controls functions of the kidney by AT1a-Ang II axis. Although NCC mRNA was also downregulated by candesartan treatment increased the responsiveness of adrenergic receptors by modulating sympathetic tone but was unable to influence the AT1a-Ang II axis in the kidney to modulate the responsiveness of AT1a mRNA. It is evident that both NCC mRNA and AT1a mRNA work with synergy in the kidney by altering renal functional capabilities at tubular glomerulus levels as supported by FENa (%) data and creatinine clearance while controlling the sympathetic tone of the kidney. It is interesting to know that both NCC mRNA and AT1a mRNA upregulated and administration of candesartan also downregulated NCC mRNA along with AT1mRNA indicating that AT1a mRNA influences NCC mRNA but unlikely true vice versa as downregulated NCC mRNA did not enhance the responsiveness of AT1a mRNA to its agonist as it does with adrenergic receptors to its adrenergic agonists. This finding is in comparison and cohesion with cell-culture-based reported data where they reported Ang II produces its action on NCC via a signaling pathway [66].
CONCLUSION
The present study concluded chronic administrations of candesartan restore the functional responsiveness of alpha-adrenergic receptors in normal and spontaneously hypertensive rats by alleviating mean arterial pressure, enhancement of renal cortical blood perfusion, increasing the functional capabilities of the kidney by modulating renal tubular and glomerular functions. Secondly, chronic administrations of candesartan in SHR downregulated the expression of AT1a mRNA and NCC mRNA in rat kidneys. It is interesting to know that blockade of AT1a mRNA by candesartan influences NCC mRNA however, the opposite is unlikely true since downregulated NCC mRNA did not enhance the responsiveness of AT1a mRNA to its agonist as it does with adrenergic receptors to its adrenergic agonists.
Supplementary Materials: The following supporting information can be downloaded at: www.mdpi.com/xxx/s1, Figure SF-1 (A-D): title; Dose-dependent curve of the renal vasoconstrictor response to graded doses of noradrenaline, phenylephrine, methoxamine and angiotensin II in Control WKY, WKY-CST, Control SHR, and SHR-CST.
ACKNOWLEDGMENTS: None
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: The animals were kept in a transit room for animals in the Animals Breeding House, Department of Pharmaceutical Sciences, Government College University Faisalabad, Pakistan, with sufficient ventilation. All animals were given a commercial brand for rat chow and water ad libitum. All in vivo study was performed after the approval of the Institutional Review Board for Animal Studies (Study No 19680/IRB No 680), Government College University Faisalabad, Pakistan.
