Archive \ Volume.11 2020 Issue 2
Efficacy of the perineural combination of dexamethasone with nalbuphine on duration of analgesia following supraclavicular brachial plexus block
Mohamed Gaber Mohamed El-Sayed1*, Fatma Mahmoud Ahmed2
1 Assistant Lecturer, Department of Anesthesia and Surgical Intensive Care, Faculty of Medicine, Zagazig University, Zagazig, Al-Sharkia Governorate, Egypt. 2 Lecturer of Anesthesia and Surgical Intensive Care, Department of Anesthesia and SurgicalIntensive Care, Faculty of Medicine, Zagazig University, Zagazig, Al-Sharkia Governorate, Egypt.

Abstract
Aim of the study: The study aimed to optimize the quality and duration of analgesia while preserving early recovery of motor function after brachial plexus block. Patients and methods: The study included 90 patients scheduled for elective upper limb surgeries using US-guided supraclavicular brachial plexus block. The patients were allocated randomly into three equal groups (n=30): (i) Group C: 30 ml bupivacaine 0.25%+2 ml 0.9% saline. (ii) Group B: 30 ml bupivacaine 0.5% +2 ml 0.9% saline. (iii) Group M: 30 ml bupivacaine 0.25%+" 4 mg dexamethasone + 10 mg nalbuphine hydrochloride (completed to 2 ml with 0.9% normal saline)". We compared the duration of postoperative analgesia along with motor block duration, opioid consumption, and complications in the first 24 hours. Results: Statistically significant prolongation in the duration of analgesia was noticed in group M with the least opioid consumption compared to group C, also motor block was resolved early in group M compared to group B. Conclusion: Combination of dexamethasone and nalbuphine to bupivacaine 0.25% can prolong the duration of analgesia while preserving the motor function after brachial plexus block.
Keywords: brachial plexus, bupivacaine, dexamethasone, nalbuphine

INTRODUCTION
There are many types of local anesthesia techniques [1, 2]. Brachial plexus block (BPB) [3]. Brachial plexus block (BPB) is a regional anesthesia technique commonly used in both inpatient and outpatient settings for upper limb surgeries and in postoperative rehabilitation. It is preferred over general anesthesia for the advantages of long-lasting pain relief, much less occurrence of nausea, vomiting, and sore throat and so, reduced recovery time [4].
The neural innervations of the upper limb allow many options for neural blocks that can be tailored to the desired level of anesthesia of the limb. The trunks and divisions of the brachial plexus are relatively close as they cross over the first rib, so the onset and quality of anesthesia are nearly fast and complete. This helped increase the popularity of the supraclavicular approach of BPB for surgeries below the shoulder [5].
Brachial plexus block using local anesthetics alone ensures good operative conditions but still has a shorter duration of postoperative analgesia. Hence, many adjuvants such as morphine [6], magnesium [7] and clonidine [8], have been added to local anesthetics in BPB to achieve fast, intense, and prolonged block; however, the results are either not conclusive or linked with side effects [9].
Nalbuphine is a 14-hydroxymorphine derivative with an agonist-antagonist effect that acts on µ (mu) receptors as an antagonist and as agonist on κ (kappa) receptors with an analgesic strength parallel to morphine while its antagonistic activity is approximately ¼th of that of naloxone. Nalbuphinehas a ceiling effect on respiratory depression not as morphine. The use of perineuralnalbuphinein brachial plexus block has shown the potentiality to extend the sensory block duration and to decrease the total analgesic consumption [10].
Perineuraldexamethasoneis thought to relieve pain via local suppression of inflammatory mediators release and also ectopic neural discharge in the nociceptive C-fibers [11]. It has been shown that the duration of postoperative analgesia could be augmented when dexamethasone is given as an adjunct for peripheral nerve blocks [12].
PATIENTS AND METHODS:
This prospective,double-blinded, randomized, controlled clinical trial was performed in Zagazig University Hospitals within the period from December 2018 to November 2019. A total of 90 patients undergoing elective below shoulders upper limb surgeries under ultrasound-guided Supraclavicular BPB were enrolled in the study. The details of the procedure were demonstrated to each patient, followed by informed consent from the patients. Patients included were aged between 21 and 60 years old, of both sexes with BMI between 18.5 and 29.9 kg/m2 and classified by the American Society of Anesthesiologist (ASA) as Class I–II. Patients with pathological coagulopathy, peripheral neuropathy, infection at the injection site or allergy to any of the studied drugs were excluded from the study.
During the preoperative visit, the visual analog scale (VAS) with ten centimeters grading (0 – no pain and 10 – worst pain imaginable) was explained to the patients. All the patients were kept fasting for about 6 hours before the procedure. An intravenous line was secured and supplemental oxygen was administered at 4 L/min through a nasal cannula. Procedural sedation had been provided using midazolam 0.01 up to 0.1 mg/kg intravenously [13]. Standard ASA monitors such as electrocardiogram, non-invasive blood pressure, and pulse oximeter were connected to the patient and baseline measures were recorded. All equipment for resuscitation were at hand and ready.
Patients were then specified randomly into three equal groups each of 30 patients using computer-generated tables:
- Group C (n=30), patients received 30 ml bupivacaine 0.25% + 2 ml normal saline.
- Group B (n=30), patients received 30 ml bupivacaine 0.5% + 2 ml normal saline.
- Group M (n=30), patients received 30 ml bupivacaine 0.25% +" 4 mg dexamethasone + 10 mg nalbuphine hydrochloride (completed to 2 ml with normal saline)".
Ultrasound-guided supraclavicular BPB was performed using an 8 to 12 MHz linear ultrasound probe (Mindray M5-Shenzhen MindrayBiomdical electronics co., LTD) placed in a sterile sheath and using the in-plane technique. The patient was kept in a supine posture with the head tilted to the opposite side of the block and after recognizing the brachial plexus at the first rib, lateral to the subclavian artery (Fig.1), skin infiltration with lidocaine 1% was done then a sterile blunt Stimuplex50 mm needle 22-gauge (B. Braun, Melsungen, AG) was directed toward the angle between the subclavian artery and first rib. Then, the anesthetic mixture "32 ml "was injected ensuring spread in the corner pocket of the plexus sheath.
Primary outcome parameters were the duration of analgesia, the total opioid consumption in the first 24 hours postoperative and duration of the motor block where secondary outcome measures were the onset of sensory and motor block and drug-related complications such as pruritus, nausea, vomiting, and sedation.
Patients were examined for the onset of a block at every 2 minutes interval till desired surgical anesthesia achieved with "time 0" is the time of completion of the injection. The onset of sensory block was designated as the time from the end of injection of the studied drugs to loss of pinprick sensation in the median, ulnar, radial and musculocutaneous nerve dermatomes, pinprick test was done using sterile 25G needle. The onset of motor block was assigned as the time from the end of the injection of the studied drugs to the loss of motor function in elbow, wrist, and fingers.
Duration of analgesia was designated as the time from the onset of sensory block to the first complaint of postoperative pain at the surgical site that had VAS ≥ 4, motor block duration was designated as the period from the onset of a motor block to the recovery of the hand or wrist mobility and was determined by asking the patients to record the exact time when they could first move the fingers of a blocked hand.
Vital signs including heart rate, blood pressure, respiratory rate, and oxygen saturation were monitored throughout the surgery. Side effects as pruritis, nausea, vomiting, sedation or pneumothorax were documented. Postoperative pain was assessed at a 1-hour interval for the first 8 hours, then every 4 hours up to the end of the first 24 hours after the procedure. When rescue analgesia was required (VAS ≥4), fentanyl 25 μg intravenous increments were administered to the patient as needed up to 200 μg/hour [14]. The total dose of fentanyl in the first 24 hours postoperative was recorded for analysis.
RESULTS
One hundred and fifteen patients (115) were enrolled to participate in this clinical study, 8 patients were precluded from the study due to failure to achieve a complete block within 30 minutes and general anesthesia was conducted to complete the surgery as needed, 7 patients refused to participate, while 10 patients did not meet the inclusion criteria (Fig.2). All groups were comparable according to their demographic data including age, height, sex, weight, BMI, ASA classification and period of surgery (Table 1). Perioperative hemodynamics; heart rate, blood pressure, peripheral oxygen saturation, and respiratory rate were also comparable between the studied groups.
As regards sensory block onset, there was no significant difference between the 3 groups, but there was a statistically significant difference between groups regarding the onset of a motor block which was shorter in group B then group M. Concerning the duration of motor block, there was a high statistically significant difference between groups. The longest duration occurred in group B(1142.4 ± 78.4) minutes, compared to group M (783.8± 54.8) minutes and group C (622.6± 57.6) minutes (Table 2).
Given the postoperative analgesia, it was significantly longer in group B and group M, compared to group C (1267.1± 93.5and 1203.1 ± 117.2vs728.8± 47.5 minutes) respectively with no statistically significant difference between group B and group M. While the total fentanyl consumption in the first 24 hours postoperative was statistically significantly lower in group M compared to other groups, with no statistically significant complications among the studied groups.
DISCUSSION
This study was done to assess the efficacy of combining dexamethasone with nalbuphine to bupivacaine 0.25% for BPB regarding the beginning of sensory and motor block, duration of motor block and postoperative analgesia, the total fentanyl consumption in the first 24 hours and any associated complications.
Regarding the onset of sensory block, there was no statistically significant difference among the groups. While the onset of motor block was shorter in bupivacaine 0.5% group than other groups. These results referred that neither dexamethasone nor nalbuphine can hasten the onset of block. These results are in agreement with the results of Das et al. and Gupta et al. [9, 15] in their studies on nalbuphine as an adjuvant to bupivacaine0.5% in BPB. Also with the results of Parrington et al.[16] in their study on dexamethasone added to mepivacaine in supraclavicular BPB.
However, Chiruvella et al., [17] in their studies evaluating the analgesic effect of nalbuphine with bupivacaine in BPB found that it significantly shortened the onset of sensory and motor block. Biradar et al. [18] also showed that the onset of both motor and the sensory block was shorter in the dexamethasone group in their comparative study of combining dexamethasone to lidocaine in BPB.
The duration of analgesia was extended in the dexamethasone plus nalbuphine group together with the least fentanyl consumption in the first 24 hours. This was highly statistically significant when compared to bupivacaine 0.25% alone. Ghanem et al. [19] found also that combining epidural dexamethasone with intrathecal nalbuphine could prolong the duration of analgesia in lower abdominal surgeries. These results are in alignment with the meta-analysis done by Huynh et al. [12] to evaluate the effect of mixing dexamethasone with a local anesthetic on peripheral nerve blocks in adults, also with the results of Gupta et al. [15] and Das et al. [9]. Nazir& Jain [20] and Abdelhaq et al. [10] also gave the same result with nalbuphine but with larger dose "20 mg" than our study "10 mg".
Comparing bupivacaine 0.5% group"B" with dexamethasone plus nalbuphine group "M", both extend the postoperative analgesia with no statistically significant difference between them. However, group M characterized by earlier recovery of motor function with much less opioid consumption. This is clinically beneficial in postoperative rehabilitation following orthopedic surgeries, which require early mobility to avoid joint stiffness and so, guarantee better surgical results and less hospital burden.
The mechanism of the analgesic effect of perineural nalbuphine can be attributed to various factors also such as the possible existence of peripheral opioid receptors permitting for the analgesic effects of opioids [21]. Also, opioids could exert local anesthetic action via the closure of sodium channels embedded in the nerve membrane [22]. At last, perineural nalbuphine analgesia can be referred to as its systemic absorption [23].
The mechanism of dexamethasone's influence in extending the analgesia period is not yet clear and is believed to be related to many factors. Possible explanations refer to some sort of vasoconstriction which could decrease the absorption of local anesthetics [24], others suppose that the suppression of the synthesis and release of the inflammatory mediators could interrupt the transmission of pain signals in the unmyelinated C-fibers [11, 25].
CONCLUSION
From the results of our study, we can conclude that the addition of 10 mg of nalbuphine hydrochloride with4 mg of dexamethasone to bupivacaine 0.25% in brachial plexus block has the advantages of extending the duration of postoperative analgesia and reducing postoperative opioids requirements while preserving early recovery of motor function when compared to bupivacaine 0.5%.
Conflict of interests
The authors declare no conflict of interest.
REFERENCES
- Tawfik S A, Abd Elaleem M A, Hassan M M, Mohamed A H, Elhamoly S M. Dexmeditomedine versus Clonidine as an adjuvant to Levobupivacaine in Paravertebral analgesia for acute post mastectomy pain. J. Adv. Pharm. Edu. Res. 2018; 8(3): 16-19.
- ElDegwy M, Omar A, Elasheery A O, Shaaban M A. Strategic management of deep sternal wound infection using vacuum assisted closure system. J. Adv. Pharm. Edu. Res. 2017; 7(4): 443-449.
- Jouybar R, Emami S, Kamalipour H, Khademi S. Effects of Pregabalin on Postoperative Pain and Agitation following Coronary Artery Bypass Grafting: Randomized Clinical Trial. Pharmacophores. 2017; 8(4): 55-61.
- Bruce, Benjamin G., Blaine, T. A., &Wesner, L. V.Brachial plexus blocks for upper extremity orthopedic surgery. JAAOS-Journal of the American Academy of Orthopaedic Surgeons, 2012, 20.1: 38-7.
- Perlas, A., Lobo, G., Lo, N., Brull, R., Chan, V. W., &Karkhanis, R. Ultrasound-guided supraclavicular block: outcome of 510 consecutive cases, 2009; 171-176.
- Murphy DB, McCartney CJ, Chan VW. Novel analgesic adjuncts for brachial plexus block: A systematic review. AnesthAnalg 2000;90:1122-8.
- Goyal P, Jaiswal R, Hooda S, Goyal R and Lal J.Role of magnesium sulphate for brachial plexus analgesia. Internet J Anesthesiol,2008, 21(1).
- Kohli S, Kaur M, Sahoo S, Vajifdar H, Kohli P. Brachial plexus block: Comparison of two different doses of clonidine added to bupivacaine. J Anaesthesiol Clin Pharmacol, 2013;29:491-5.
- Das, A., RoyBasunia, S., Mukherjee, A., Biswas, H., Biswas, R., Mitra and Mandal, S. K. Perineuralnalbuphine in ambulatory upper limb surgery: A comparison of effects of levobupivacaine with and without nalbuphine as adjuvant in supraclavicular brachial plexus block. Anesthesia: Essays and Researches, 2017;11(1), 40-46.
- Abdelhaq MM, Elramely MA. Effect of nalbuphine as an adjuvant to bupivacaine for ultrasound-guided supraclavicular brachial plexus block. Open JAnesthesiol; 2016; 6:20–6.11.
- Devor M, Govrin-Lippmann R, Raber P. Corticosteroids suppress ectopic neural discharge originating in experimental neuromas. Pain. 1985;22:127–137.
- Huynh, T. M., Marret, E., and Bonnet, F. Combination of dexamethasone and local anesthetic solution in peripheral nerve blocks: A meta-analysis of randomized controlled trials. European Journal of Anesthesiology (EJA), 2015; 32(11), 751-758.
- Butterworth J F, Mackey DC, and Wasnick J D. Local Anesthetics, Morgan and Mikhail’s clinical anesthesiology, 5th ed, USA, McGraw-HillEducation, 2013; Ch16, 263-79.
- Abdallah, F. W., Johnson, J., Chan, V., Murgatroyd, H., Ghafari, M., Ami, N. and Brull, R. Intravenous dexamethasone and perineural dexamethasone similarly prolong the duration of analgesia after supraclavicular brachial plexus block: Regional anesthesia and pain medicine,2015, 40(2), 125-132.
- Gupta, K., Jain, M., Gupta, P. K., Rastogi, B., Zuberi, A., &Pandey, M. N. Nalbuphine as an adjuvant to 0.5% bupivacaine for ultrasound-guided supraclavicular brachial plexus blockade. Indian Journal of Pain, 2016;30(3), 176.
- Parrington SJ, O’Donnell D, Chan VW, Brown-Shreves, D., Subramanyam, R., Qu, M., &Brull, R. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. RegAnesth Pain Med; 2010;35:422–426.
- Chiruvella, S., Konkyana, S. K., Nallam, S. R., &Sateesh, G. Supraclavicular brachial plexus block: Comparison of varying doses of nalbuphine combined with levobupivacaine: A prospective, double-blind, randomized trial. Anesthesia, essays and researches, 2008;12(1), 135.
- Biradar, P. A., Kaimar, P., &Gopalakrishna, K. Effect of dexamethasone added to lidocaine in supraclavicular brachial plexus block: A prospective, randomized, double-blind study. Indian Journal of Anesthesia, 2013; 57(2), 180.
- Ghanem, Mohamed, et al. Efficacy of epidural dexamethasone combined with intrathecal nalbuphine in lower abdominal oncology operations. Anesthesia, essays and researches, 2019,13.3: 560.
- Nazir, N., & Jain, S. Randomized controlled trial for evaluating the analgesic effect of nalbuphine as an adjuvant to bupivacaine in supraclavicular block under ultrasound guidance. Anesthesia, essays and researches, 2017; 11(2), 326.
- Stein C. opioid receptors on peripheral sensory neurons. AdvExp Med Biol.; 2003; 521: 69–76.
- Likar R, Koppert W, Blatnig H, Chiari F, Sittl R, Stein C, SchäferM. Efficacy of peripheral morphine analgesia in inflamed, noninflamed and perineural tissue of dental surgery patients. J PainSymptom Manage; 2001; 21: 330–7.
- Sehgal N, Smith HS, Manchikanti L. Peripherally acting opioids and clinical implications for pain control. Pain Phys.; 2011;14(3):249–58.
- Kawanishi R, Yamamoto K, Tobetto Y, et al. Perineural but not systemic low-dose dexamethasone prolongs the duration of interscalene block with ropivacaine: A prospective randomized trial. Local RegAnesth.2014;7:5–9.
- Johannsson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive c-fibers. Acta Anaesthesiol Scand.1990;34:335–338.
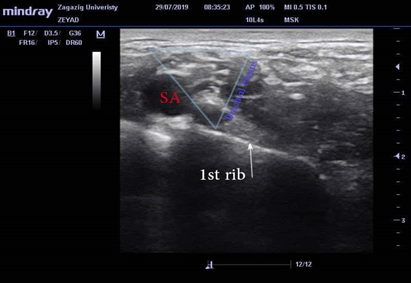
Figure 1: Ultrasound image of the brachial plexus in the supraclavicular area, SA stands for the subclavian artery.
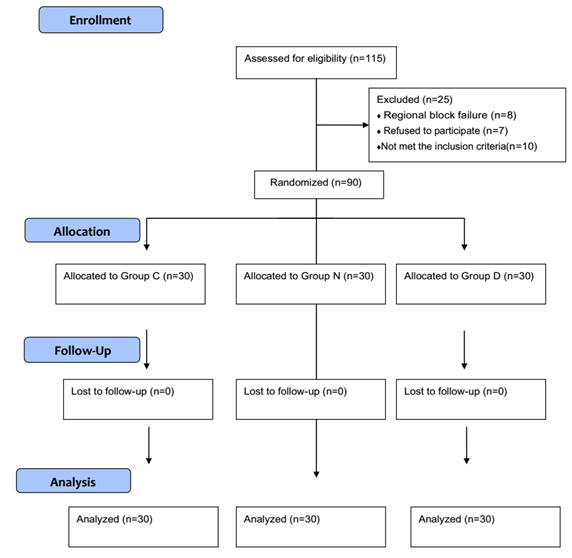
Figure 2: Consort flow chart.
|
Table 1: Demographic features of the studied groups |
|||||||||||||||
|
Item |
Group C (N=30) |
Group B (N=30) |
Group M (N=30) |
χ2 /KWt
|
P-value |
||||||||||
|
|
No. |
% |
No. |
% |
No. |
% |
|
|
|||||||
|
Age (years) |
|||||||||||||||
|
Mean ± SD |
39.73 ± 9.77 |
38 ± 10.85 |
39.17 ± 12.4 |
0.450 |
0.799(NS) |
||||||||||
|
Sex |
|||||||||||||||
|
Male/Female |
17/13 |
56.7/43.3 |
16/14 |
53.3/46.7 |
16/14 |
53.3/46.7 |
0.090 |
0.956(NS) |
|||||||
|
Weight (kg) |
|||||||||||||||
|
Mean ± SD |
82.7 ± 7.9 |
80.2 ± 8.1 |
80.73 ± 8.19 |
1.48 |
0.477(NS) |
||||||||||
|
Height (cm) |
|||||||||||||||
|
Mean ± SD |
161.74 ± 5.9 |
163.1 ± 8.4 |
172.28 ± 7.27 |
0.813 |
0.666(NS) |
||||||||||
|
BMI (kg/m2) |
|||||||||||||||
|
Mean ± SD |
27.5 ± 1.8 |
26.7 ± 1.76 |
26.99 ± 1.62 |
4.54 |
0.103(NS) |
||||||||||
|
ASA |
|||||||||||||||
|
Grade I / II |
16/14 |
53.3/46.7 |
17/13 |
56.7/43.3 |
14/16 |
46.7/53.3 |
0.090 |
0.956 (NS) |
|||||||
|
Duration of surgery (minutes) |
|||||||||||||||
|
Mean ± SD |
129.27 ± 26.3 |
131.3 ± 27.33 |
134.9 ± 21.92 |
1.62 |
0.445(NS) |
||||||||||
χ2: chi-square test
KWt: Kruskal Wallis test.
P < 0.05 is significant.
NS: Not significant.
|
Table 2: BPB characteristics among the studied groups |
|||||
|
Item |
(N=30) |
(N=30) |
(N=30) |
KWt |
P-value |
|
Onset of sensory block(minutes) |
|||||
|
Mean ± SD |
15.5 ± 1.94 |
14.46 ± 2.04 |
14.6 ± 2.17 |
4.15 |
0.125(NS) |
|
Onset of motor block (minutes) |
|||||
|
Mean ± SD |
22.3 ± 2.10 |
18.76 ± 2.52 |
20.60± 2.51 |
24.76 |
0.000*(HS) |
|
Duration of motor block(minutes) |
|||||
|
Mean ± SD |
622.6± 57.6 |
1142.4 ± 78.4 |
783.8± 54.8 |
77.42 |
0.000*(HS) |
|
p-value of MWt |
---- |
0.000*a |
0.000*b |
|
|
|
---- |
----- |
0.000c |
|||
|
Duration of analgesia (minutes) |
|||||
|
Mean ± SD |
728.8± 47.5 |
1267.1± 93.5 |
1203.1 ± 117.2 |
60.82 |
0.000*(HS) |
|
p-valueof MWt |
---- |
0.000*a |
0.000*b |
|
|
|
---- |
----- |
0.070 c |
|||
|
Total fentanyl dose in the first 24 hours (μg) |
|||||
|
Mean ± SD |
165.8± 38.5 |
49.83± 23.87 |
26.67± 15.9 |
67.25 |
0.000*(HS) |
|
p-value of MWt |
---- |
0.000*a |
0.000*b |
|
|
|
---- |
----- |
0.000*c |
|||
KWt: Kruskal Wallis test: for comparison between 3 groups
MWt: Mann Whitney test for comparison between 2 groups
a; comparison between Bupivacaine 0.25% and Bupivacaine 0.5% group.
b; comparison between Bupivacaine 0.25% and Dexamethasone + Nalbuphine group.
c; comparison between Bupivacaine 0.5% and Dexamethasone + Nalbuphine group.
*P < 0.05 is significant. HS: highly-significant.
NS: non-significant.
