Archive \ Volume.11 2020 Issue 2
Review of herb supplement use in type 2 diabetes
Khalid Z Alshali
Department of Internal medicine, Endocrinology Unit, Faculty of Medicine, King Abdul Aziz University, Jeddah, Kingdom of Saudi Arabia.

Abstract
Diabetes mellitus is a major metabolic disorder and has a big proportion of affliction in the world which is recognized for many complications, as diabetic nephropathy, neuropathy, and retinopathy. Fenugreek, cinnamon, bitter melon, Gymnema Sylvestre, ginger, olive leaf extract, and berberine are the most common plants for treating diabetes. These plants contain many important substances as alkaloids, flavonoids, etc, which are also rich in soluble fibers that help decrease blood sugar by hindering the assimilation and retention of carbohydrates. Fenugreek could improve metabolic symptoms types by bringing down blood glucose and improve glucose tolerance.
Keywords: Fenugreek, cinnamon, bitter melon, Gymnema Sylvestre, ginger, olive leaf extract, berberine, diabetes mellitus

INTRODUCTION
Diabetes mellitus (DM) is commonly endocrine disorder and affects more than 400 million people which accounts for 9.1% of the world population. Its prevalence is rapidly increasing and it is estimated that by 2040, 642 million people in the world will be affected by diabetes. DM caused by the reduction of insulin production through the pancreas which results in increase glucose concentrations in blood [1-3].
Diabetes is an inveterate disease that reduces the quality of life, increases the risk of morbidity and mortality, and can damage many of the body systems; particularly the blood vessels and nerves. It is a common and serious metabolic disorder throughout the world [4-6].
Widely structures' ranges of constituent's plant are active hypoglycaemic principles. Ayurveda could use in treating many human diseases. More than 45000 claimed to have been used as medicinal plants in India [7].
The present research aims to review various plant species as fenugreek, cinnamon, bitter melon, Gymnema Sylvestre, ginger, olive leaf extract, and berberine and have used as traditional medicine and have shown hypoglycaemic activity.
Fenugreek (Trigonella foenum graecum)
Fenugreek (Trigonella)is means "small triangle" related to yellowish-white triangular flower [8]. The name of Foenum-graecum (Greek hay) is a native plant in the Mediterranean region (Asia). Fenugreek kinds are known in the world (Table 1). More than 260 species are available in the Trigonella genus [9].
|
Table 1: Fenugreek names (Trigonella foenum graecum) [10]. |
|
|
Language |
Common Names |
|
Hindi |
Methi, Saag methi, Kasuri methi |
|
English |
Fenugreek |
|
French |
Fenugreec, Trigonelle |
|
Galician |
Alforfa |
|
German |
Bockshornklee, Griechisch Heu |
|
Georgian |
Solinji, Chaman |
|
Japanese |
Koruha, Fenu-guriku |
|
Dutch |
Fenugriek |
|
Romanian |
Molotru, Molotru comun, Schinduf |
|
Assamese |
Methi, Mithi |
|
Sanskrit |
Methika |
Chemical constituents of fenugreek
Fenugreek seeds are rich sources of soluble dietary fiber [11]. 100 g in seeds give over 65% of daily recommended dietary fiber. Fenugreek contains saponins, hemicelluloses, adhesive, tannins and gelatin, and these mixes help to diminish the levels of low-density lipoprotein-cholesterol (LDL) in blood by hindering bile salts reabsorption in the colon [12]. The seed of fenugreek has a high proportion of protein ranging from 20 to 30% as well as an amino acid, 4-hydroxy isoleucine, which potentiates glucose-induced insulin secretion [13]. Fenugreek seed contains a high amount of vitamins like vitamin A, B1, B2 and C (0.003, 0.43, 0.36 and 12 to 43 mg/100g), potassium (603.0 mg/100g), magnesium (42.0 mg/100g), calcium (75.0 mg/100g), zinc (2.4 mg/100g) and iron (25.8 mg/100g) [14]. Fenugreek endosperm contains 35% alkaloids, primarily trigonelline. Flavonoid constitutes more than 100 mg/g of fenugreek seed [15].
Antidiabetic effect of fenugreek
Fenugreek seed extracts have antidiabetic potential by delaying both gastric emptying time and rate of glucose absorption and reduced uptake of glucose is due to the high content of fiber [16]. Pancreatic function tissues protected β cells, elevated serum insulin by β cells regeneration or stimulation of insulin release by the existing β islet cells [17].
Insulin sensitivity, improving insulin action at the cellular level and improved HbA1c level by utilizing glucose in peripheral tissues thereby maintain normal blood glucose level [18].
Free radicals role in diabetes pathogenesis and oxidative stress coexists by reduced antioxidant status as antioxidant activity [18] which could prevent diabetes pathogenesis. Solid-state bioconversion for fenugreek substrate through Rhizopus oligosporus shown an increase of natural α-amylase inhibitors associated and increasing phenolic antioxidants and reducing glycemic index. A specific amino acid called 4-hydroxy isoleucine represents about 80% of free amino acid in fenugreek seeds. 4-hydroxy isoleucine reported to suppressing of progression of diabetes 2 in the mice model [19].
Fenugreek oil has antidiabetic effects related to immunomodulatory action and insulation in alloxanized rats. Daily oral by fenugreek steroids showed reducing of glucose blood and improving insulin-immunoreactive β cells [20].
Fenugreek role in diabetes pathogenesis and complications
Important carbohydrate metabolic enzymes were altered in Streptozotocin inducing diabetic rats. Extract of Trigonella foenum-graecum and Psoralea corylifolia seeds in a compound manner (1:1) recovery activities of liver enzymes and, therefore, they reported that the extract corrected the abnormal metabolism [21].
Diabetic retinopathy and neuropathy both are common complications of diabetes. In one study, fenugreek seeds show and effective in preventing retinopathy [20]. Neuroprotective effects in fenugreek seed powder might result in decreasing in hyperglycemia and oxidative stress, [22].
Cinnamon (Cinnamomum)
Cinnamon is an ancient spice utilized in several cultural practices and using in cooking, furthermore become popular for potential health outcomes because of medicinal effects of cinnamon is declared also its antimicrobial, antioxidant, antitumor, blood pressure-lowering, cholesterol-lowering, and gastroprotective characteristics [23].
Chemical constituents of Cinnamon
Cinnamon has been utilized for centuries as a spice that adds flavor and aroma to food. The chemical composition of cinnamon is as follows: moisture (6.5-11.9%), crude fiber (12.0-28.8%), total carbohydrates (6.9-32.0%), protein (3.1-3.4%), and volatile oil (0.5- 5.1%) [24]. The volatile oil between these parts has the greatest trade value. The volatile components of cinnamon are classified in general to sesquiterpenes and phenylpropenes. The major component in cinnamon bark volatile oil is cinnamaldehyde shown in Figure (1), whereas, that the main component in cinnamon leaf oil is eugenol [25].
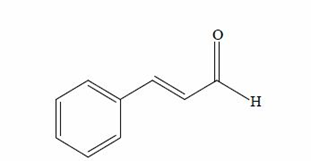
Figure 1. Cinnamaldehyde from Cinnamomum zeylanicum
Mechanisms of hypoglycemic activity of cinnamon
The mechanism of cinnamon in its antidiabetic activity and perhaps the effect of action at the pathway of insulin (Figure 2).
Cinnamtannin is a proanthocyanidin isolated from cinnamon which activates the phosphorylation of the insulin receptor β-subunit on adipocytes as well as other insulin receptors [26].
Cinnamon increases the quantity of glucose transporter 4 receptors as well as insulin receptors (IR) and insulin receptor substrates [27] thereby making it easier for glucose entry into cells. Shen et al. [28] explained that the extracts of Cinnamomum zeylanicum increase GLUT4 to the cell membrane of brown adipose tissue and muscle in a dose-contingent way. Anand et al. [29] showed a similar result of greater membrane translocation of GLUT4 in cinnamon treated rats than in controls.
Plaisier et al. [30] demonstrated cinnamaldehyde elevates the glucose transporter-1 (GLUT-1) mediated glucose absorption in a dose-dependent fashion in the L 929 fibroblasts. Meanwhile, in the presence of glucose shortage in the medium, cinnamaldehyde decreased the GLUT 1 mediated glucose absorption. Hlebowicz et al in 2009 demonstrated a dose-dependent reduction of serum insulin concentrations and an increase in glucagon-like peptide 1(GLP-1) with cinnamon treatment. Improving glucose transport across cell membrane reducing insulin resistance [31].
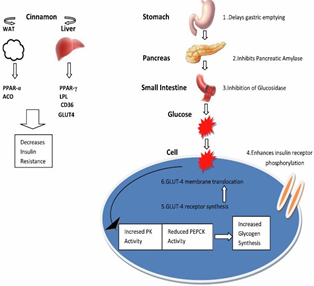
Figure 2. Mechanisms of cinnamon hypoglycemic activity.
Cinnamon influences
Strong proof indicates that cinnamon contains polyphenols compounds that appear to have insulin-like activity in rats, with type 2 diabetes. Moreover to find the molecular basis for the insulin-like activity of cinnamon, doubly linked procyanidin type-A polymers were used by Cao et al. [32] for illustrated cinnamon polyphenol compounds on the regulation of three of the proteins connected in the insulin signal transduction pathway, utilizing mouse 3T3-L1 adipocytes. Based on this investigation, it could be suggested that the cinnamon polyphenol compounds activate the insulin receptor (IR) by increasing their tyrosine phosphorylation activity and by reduced phosphatase activity [33].
Cinnamon polyphenol compounds increase the quantity of anti-inflammatory protein in the cells. These activities may lead to additional active glucose transport and use. Moreover, cinnamon polyphenols may supply one of the molecular bases for the useful influence of cinnamon, in making it better for people with diabetes by downregulating the synthesis of pro-inflammatory cytokines [33].
Bitter melon (Momordica charantia)
Bitter melon was used as food and medicine for a long time. Many plants are utilized as foods and medicines. These plants tend to be supportive, tonic and nourishing in nature These plants work effectively but seldom have strong hypoglycemic characteristics or powerful influences on affecting the reproduction tract and lowering blood sugar [34].
Momordica charantia or Bitter Melon belongs to family Cucurbitaceae. The bitter melon plant is used traditionally as both food and medicine. Bitter melon has been used for a long time as a hypoglycemic agent, where the plant extract has been regarded as vegetable insulin. A part of vegetable insulin bitter melon fruit is also used as tonic, stomachic, stimulant, emetic, antibilious, laxative and alterative. The fruit has various useful effects on the treatment of gout, rheumatism and even in curing diseases of spleen and liver [35].
Chemical constituents
Momordica charantia consists of the following chemical constituents those are alkaloids, momordicin and charantin (Figure 3) وalso, more alkaloids [36]. Momordica charantia had contained glycosides, antinutritional factors, reducing sugars, resins, a natural antioxidant, essential oil, and free acids [37] and leaves are rich in minerals like calcium, magnesium, potassium, phosphorus, and iron; Momordica charantia and leaves are a large source of B vitamin complex [38].
The chemical constituents from Momordica charantia fruit as moisture, protein and lipids were 93.2, 18.02 and 0.76%, respectively, on a dry weight basis [39]. 45% of the seed is made up of oils (63–68% eleostearic acid and 22–27% stearic acid) [40]. Moreover, various glycosides were separated from the stem (Momordica charantia) [41] and Momordica charantia fruit [42] and are gathered in the genera of cucurbitane-type triterpenoids. Specifically, four triterpenoids have AMP-activated protein kinase activity which is a proposed hypoglycaemic mechanism of Momordica charantia [43].
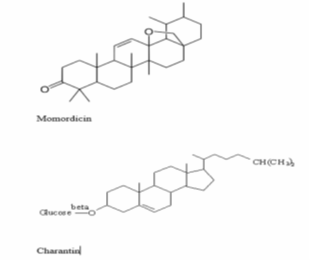
Figure 3: Chemical structure of momordicin and charantin
Regular use of bitter melon to control diabetes
Aswar and Kuchekar [44] proved that the anti-diabetic activity of Momardica charantia fruit extracts establishing the scientific basis for the utility of this plant in the treatment of diabetes. Bitter gourd contains bioactive compounds that activate a protein called AMPK (AMP-activated protein kinase α), which enables glucose uptake processes which lead to lowering glucose in the blood in patients with diabetes [45].
Bitter melon contains lectin, a bioactive compound that has an insulin-like activity which maybe because of its link together with 2 insulin receptors. Lectin lowers blood glucose concentrations through its action on peripheral tissues and, similar to insulins influence in the brain, reducing appetite. Also, the charantin is a powerful hypoglycemic agent of mixed steroids which are occasionally used in reducing the blood sugar levels [37, 46].
Gymnema Sylvestre (Gurmar)
Gymnema Sylvestre shows a wide range of therapeutic influences as an active natural therapy for diabetes, as well as hypercholesterolemia, cardiovascular diseases, infections, indigestion, and constipation. Gymnema Sylvestre has potential when it comes to treating diabetes as it shows good effects on blood sugar control, and stimulates regeneration of the pancreas. The Gymnema Sylvestre extract helps decrease body weight and improves lipid profile and for that reason is used in dietary supplements [47].
Mechanism action of Gymnemic acids
Mechanism action for the drug is through stimulation of insulin secretion from the pancreas Figure (4). As well it delays glucose absorption in the blood by attaching to receptors present in the external layer of the intestine, thereby preventing the absorption of sugar molecules by the intestine, leading to a reduction of sugar blood [48].
Hypoglycemic influence of gymnemic acids results from a cascade of events firstly from modulation of incretin activity which increases insulin excretion and release. Gymnemic acids decrease glucose and fatty acid assimilation in the small intestine and interfere in the ability of receptors in the mouth and intestine to the sensation of sweetness. [49]. Gymnemic acid has been shown to interact with GAPDH, a major enzyme in the glycolysis cycle [50]. Acyl moieties present in gymnemic acids play a significant role in GA-promoted smearing of GAPDH and G3PDH and play a significant role in the antihyperglycemic activity of GA derivatives [51].
Antidiabetic characteristics for Gymnema Sylvestre
A study to estimate the antioxidant activity of Gymnema leaf extract and the role of antioxidants in diabetic rats was carried out by Kang et al. [52] utilizing the ethanolic extract. Several antioxidant assays. Furthermore, liquid chromatography/mass spectrometry analysis revealed the presence of antihyperglycemic compounds like gymnemagenin and gymnemic acids in G. Sylvestre extract and the level of lipid peroxidation was reduced by 31.7% in serum, 9.9% in liver, and 9.1% in the kidney in diabetic rats fed with the ethanolic extract. [53].
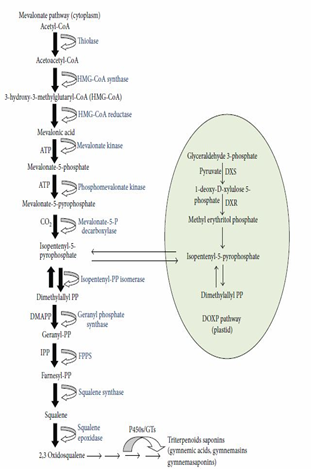
Figure 4: Hypothetical pathway of Gymnemic acid biosynthesis.
Ginger (Zingiber officinale)
It is presumed that oxidative stress plays a significant role in the secondary complications of diabetes. Finally, ginger could be lowering glycemic potential and reduce diabetic complications [54].
Bioactivity of ginger
Ginger contains many chemical constituents and could be presented as a powder [55]. The ginger chemical components could be volatile or non-volatile, the latter being responsible for the characteristic smell and taste of ginger. Among the volatile oil, compounds are sesquiterpene hydrocarbons like zingiberene, curcumin, and farnesene [56, 57]. Both gingerols and shogaols are very significant phenolic compounds since they have pharmacological characteristics that are useful to health as shown in Figure (5) [58].
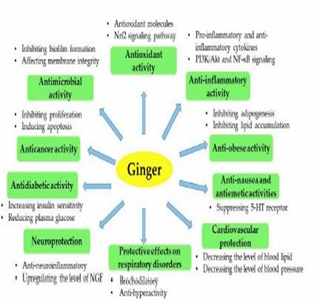
Figure 5. An overview of the bioactivities of ginger
Mechanism of action of Ginger
Diabetes mellitus is recognized as a severe metabolic disorder that is caused by insulin shortage and/or insulin resistance, resulting in elevated blood glucose levels. Figure (6) summarizes the anti-hyperglycemic mechanisms and protective effect of ginger. The high glucose levels in the blood could accelerate protein glycation and the process of formation of advanced glycation end products (AGEs) [59, 60].
In one study, 6-gingerol was found to enhance glucose-encouraged insulin secretion and improve glucose tolerance in type 2 diabetic mice by elevating glucagon-like peptide 1 (GLP-1). 6-gingerol therapy activated glycogen synthase 1 and elevated cell membrane presentation of glucose transporter type 4 [61]. Furthermore, ginger extract therapy improves insulin sensitivity in rats with metabolic syndrome which might have been closely connected to the energy metabolism enhancement induced by 6-gingerol [62]. As well ginger extract reduces retinal microvascular alterations in rats that had diabetes induced by streptozotocin. The ginger growth factor in the retinal tissue [63].
Ginger has been found to modulate insulin release. In vitro, ginger extract augmented insulin release from the pancreatic β-cell in the rat. In arsenic-induced type 2 diabetic rats, [6]-gingerol showed a protective effect on pancreatic β-cells and restored the plasma insulin level [64]. The mechanism behind this action of ginger may involve interplay with the 5-HT3 receptor [65].
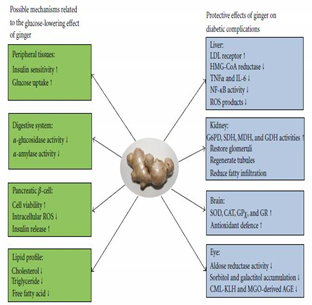
Figure 6: Summary of anti-hyperglycemic mechanism and protective effect of ginger
Olive leaf extract
Chemical constituents of olive leaf extract
Olive leaves (OLs) contain a large variety of phenols including rutin, flavones; flavan-3-ols, substituted phenols, oleoside and secoiridoid glycoside [66, 67].
Mechanism of antidiabetic action of olive leaf extract
de Bock et al. [68] reported that olive leaf extract (OLE) improves both insulin sensitivity and pancreatic β-cell secretory capacity after oral glucose challenge on overweight males. In another study, the treatment of diabetic rats with OLE significantly decreased HbA1c. However, Wainstein et al. in this study [69] did not measure the physical activities and diet type of the participants, so the independent effect of OLE alone could not be determined.
A recent study showed a correlation in the OLE treated group when α-glucosidase and α-amylase enzyme activities were compared with blood glucose levels [70]. It was found that the blood glucose levels were markedly attenuated while the enzyme activities decreased in the OLE group. OLE is considered to be effective in decreasing blood glucose levels by (i) inhibiting activity of carbohydrate digestive enzymes, α-glycosidase, and α-amylase, or (ii) downregulating gene expressions of these enzymes. Furthermore, this study demonstrated partial positive immunoreaction for insulin in β-cells through immunohistochemical analysis corroborating OLEs effect on insulin production seen in the de Bock et al study.
Antidiabetic medications such as acarbose, an a-glucosidase inhibitor, cause undesirable symptoms due to undigested starch in the colon [71]. Thus, tending toward alternative α-glucosidase inhibitors that are derived from natural sources and nutrients may be more effective, safe, tolerable and cheaper.
Berberine
Berberine is a traditional Chinese medicine extracted from Chinese rhizomacoptidis, cortex phellodendri, berberis, and other plants. In modern years there has been an increased number of studies in China to examine the treatment effects of Berberine in patients with diabetes. Changrong et al. [72] performed a randomized control trial of Berberine versus metformin and found Berberine effectively lowered blood glucose.
Antidiabetic effect of berberine
Berberine enhances glucose metabolism in type 2 diabetes mellitus by inhibition of liver gluconeogenesis through activation of AMPK (Adenosine Monophosphate Activated Protein Kinase) and also it ameliorates insulin sensitivity. Inhibition of liver gluconeogenesis leads to a reduction of fasting blood glucose levels. This is an insulin-independent action and includes mitochondrial inhibition by berberine [73].
Berberine activates AMPK leading to its increased phosphorylation resulting in consistent elevation of AMP/ATP ratio and reduction in the consumption of oxygen. Mitochondrial inhibition and an increase in AMP/ATP ratio result from AMPK activation by berberine [74]. Another mechanism explaining the antihyperglycemic action of berberine is its effect in increasing GLP 1 biosynthesis and its release [75, 76].
Moreover, berberine is recognized to modulate insulin secretion by pancreatic beta cells [77]. Metabolic syndrome occurs when AMPK regulated pathways are switched off and produce hyperglycemia, hyperlipidemia, hypertension, obesity, and inflammation. Only a few compounds can activate AMPK. Berberine is one of them and forms the basis for the treatment of metabolic syndrome [78]. Berberine also suppresses proinflammatory responses [79]. This action is mediated via AMPK activation as well.
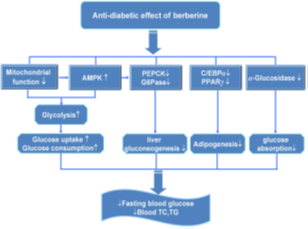
Figure 7. Anti-diabetic effect of berberine. Ming Zhang [80].
CONCLUSION
Diabetes mellitus is the most common endocrine disorder, Thus, therapy for diabetes mellitus using plant-derived compounds that are acceptable and do not need pharmaceutical synthesis to appear very attractive. All the herbal drugs mentioned in this review exhibit important clinical activity. Therefore, it would be recommended to carry out more research work on different plant species and their active compounds to provide evidence that certain traditional herbal therapies are beneficial in lowering blood glucose in patients with diabetes.
REFERENCES
- World Health Organization. 2017. WHO fact sheet, [Online] 2016 [Cited 2017 May 30]. Available from: URL: http://www.who.int/ media.
- Khazaii R, Kamareh S. Relationship between diabetes and periodontal disease: a review of literature. Ann. Dent. Spec. 2018; 6(1):57-60.
- Aziz N, Wal A, Wal P, Pal RS. Preparation and Evaluation of the Polyherbal Powder: The Nature’s Pharmacy for the Treatment of Diabetes Mellitus and Its Complications. Pharmacophores. 2019; 10(1):60-70.
- American Diabetes Association. Standards of Medical Care in Diabetes-2017. Diabetes Care. 2017; 40: 51-126.
- Yuniarto A, Sukandar EY, Fidrianny I, Setiawan F, Ketut I. Antiobesity, Antidiabetic and Antioxidant Activities of Senna (Senna alexandrina Mill.) and Pomegranate (Punica granatum L.) Leaves Extracts and Its Fractions. Int. J. Pharm. Phytopharm. Res. 2018; 8(3):18-24.
- Fathima HM, Thangavelu L, Roy A. Anti-diabetic activity of cassia fistula (alpha amylase–inhibitory effect). J. Adv. Pharm. Educ. Res. 2018; 8(2):12-5.
- Bordoloi, R. and Dutta, KN. A Review on Herbs Used in the Treatment of Diabetes mellitus, J Pharm Chem Biol Sci, August 2014; 2(2):86-92
- Awais A, Salem S, A, Kaiser M, Muhammad A. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi Journal of Biological Sciences. 2015; 23:1-11.
- Acharya SN, Thomas JE, Basu SK. Fenugreek: an old-world crop for the new world. Biodiversity. 2014; 7:27-30.
- Shashikumar JN, Champawat PS, Mudgal VD, Jain SK, Deepak S, Mahesh K. A review: Food, medicinal and nutraceutical properties of fenugreek (Trigonella foenum-graecum L.). International Journal of Chemical Studies. 2018; 6:1239-1245.
- Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ. Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes, Molecular and Cell Biochemistry. 2001; 224:45-51.
- Meghwal M. and Goswami TK. A review on the functional properties, nutritional content, medicinal utilization and potential application of fenugreek. Journal of Food Process Technology. 2012, 3:9.
- Isikli ND and Karababa E. Rheological characterization of fenugreek paste (cemen), Journal of Food Engineering 2015; 69:185-190.
- Al Jasass FM and Al Jasser MS. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Scientific World Journal 2012. http://dx.doi.org/10.1100/2012/859892.
- Naidu MM, Shyamala B, Naik JP, Sulochanamma G, Srinivas P. Chemical composition and antioxidant activity of the husk and endosperm of fenugreek seeds. LWT – Food Science and Technology. 2011; 2:451-456.
- Patil, P. M., Chaudhari, P. D., Duragkar, N. J. and Katolkar, P. P. “Formulation of an anti-diabetic liquid preparation of Gymnema Sylvestre and qualitative estimated by TLC,” Asian Journal of Pharmaceutical and Clinical Research, 2012, 5(1):16–19.
- Bera TK, Ali KM, Jana K, Ghosh A, Ghosh D. Protective effect of aqueous extract of seed of Psoralea corylifolia (Somraji) and seed of Trigonella foenum-graecum L. (Methi) in the streptozotocin-induced diabetic rat: A comparative evaluation. Pharmacognosy Research. 2013; 5:277-85.
- Gauttam VK and Kalia AN. Development of polyherbal antidiabetic formulation encapsulated in the phospholipids vesicle system. Journal of Advanced Pharmaceutical Technology and Research. 2013; 4:108-17.
- Randhir R and Shetty K. Improved alpha-amylase and Helicobacter pylori inhibition by fenugreek extracts derived via solid-state bioconversion using Rhizopus oligosporus. Asia Pacific Journal of Clinical Nutrition. 2007; 16:382-92.
- Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, Pillai KK. A pharmacological appraisal of medicinal plants with antidiabetic potential. Journal of Pharmacy and Bioallied Sciences. 2012; 4:27-42.
- Bera TK, Ali KM, Jana K, Ghosh A, Ghosh D. Protective effect of aqueous extract of seed of Psoralea corylifolia (Somraji) and seed of Trigonella foenum-graecum L. (Methi) in streptozotocin-induced diabetic rat: A comparative evaluation. Pharmacognosy Research. 2013; 5:277-85.
- Kumar P, Kale RK, Baquer NZ. Antihyperglycemic and protective effects of Trigonella foenum graecum seed powder on biochemical alterations in alloxan diabetic rats. European Review for Medical and Pharmacological Sciences. 2012; 16:18-27.
- Bandara, T., Uluwaduge, I., Jansz. E. R. Bioactivity of cinnamon with special emphasis on diabetes mellitus: A review, International Journal of Food Sciences and Nutrition, 2011; 1–7
- Leela, N.K. In: V.A. Parthasarathy, B. Chempakam, T.J. Zachariah (Ed.), Chemistry of Spices (CAB International, Oxfordshire, 2008, 124-145.
- Thomas, J., Duethi, P.P. In K.V. Peter (Ed.), Handbook of herbs and spices (Woodhead Publishing Ltd, England, 2001, 143-153.
- Taher M, Fadzilah Adibah AM, Mohomad RS. A proanthocyanidin from Cinnamomum zeylanicum stimulates phosphorylation of insulin receptors in 3 T3-L1 adipocytes. J Teknologi. 2006;44:53–68.
- Shen YIto Y, Muraki E, Honoso T, Seki T. Cinnamon extract enhances glucose uptake in 3 T3–L1 adipocytes and C2C12 myocytes by inducing LKB1AMP-activated protein kinase signaling. PLoS One. 2014; 9(2):87894.
- Shen Y, Fukushima M, Ito Y, Muraki E, Hosono T, Seki T. Verification of the antidiabetic effects of cinnamon (Cinnamomum zeylanicum) using insulin-uncontrolled type 1 diabetic rats and cultured adipocytes. Biosci Biotechnol Biochem. 2010;74:2418–25.
- Anand P, Murali K, Tandon V, Murthy PS, Chandra R. Insulinotropic effect of cinnamaldehyde on transcriptional regulation of pyruvate kinase, phosphoenolpyruvate carboxykinase, and GLUT4 translocation in experimental diabetic rats. Chemico-Biolog Interact. 2010;186:72–81.
- Plaisier C, Cock A, Scott J, Opejin A, Bush house KT, Salie MJ. Effects of cinnamaldehyde on the glucose transport activity of GLUT1. Biochimie. 2011;93 (2):339–44.
- Hlebowicz J, Hlebowicz A, Lindstedt S, Bjorgell O, Hoglund P, Hoist JJ. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am J Clin Nutr. 2009;89(3):815–21.
- Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys., 2007, 459, 214-222.
- Jarvill-Taylor, K. J., Anderson, R. A. and Graves, D. J. A hydroxy chalcone derived from cinnamon functions as a mimetic for insulin in 3T3-L1 adipocytes, Journal of the American College of Nutrition, 2001, 20(4): 327–336
- Abascal, K. and Yarnell, E. Using Bitter Melon to Treat Diabetes, Al Ternative and Complementary Therapie, 2005; 179-184
- Chanda, R., Samadder, A., Banerjee, J. Anti-diabetic Activity of Momordica Charantia or Bitter Melon: A Review, Acta Scientific Pharmaceutical Sciences, 2019; 3(5): 24-30.
- Braca A., Siciliano, T., D’Arrigo, M., Germanòm M.P. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoter, 2008; 79: 123-125
- Kumar, S.D., K.V. Nath, P. Yogeswaran, A. Harani, K. Sudhakar, P. Sudha, dan D. Banji. A Medical Potency of Momordica Charantia, International Journal of Pharmaceutical Sciences Review and Research, 2010, 1: 95. 1.
- Gupta, M., Sushil, S, Ajay K. G, Bhadauria, D. R. Momordica Charantia Linn. (Karela) Nature’s Silent Healer, International Journal of Pharmaceutical Sciences Review and Research., 2011., 11: 32-37.
- Yuwai KE, Rao KS, Kaluwin C. Chemical composition of Momordica charantia L. fruits. J Agric Food Chem., 1991, 39, 1762–1763.
- Chang MK, Conkerton EJ, Chapital DC. Chinese melon (Momordica charantia L.) seed: composition and potential use. J Am Oil Chem Soc., 1996, 73, 263–265.
- Chang, Chi-I., Chiy-Rong Chen, Yun-Wen Liao, Hsueh-Ling Cheng, Yo-Chia Chen, and Chang-Hung Chou. "Cucurbitane-Type Triterpenoids from Momordica c harantia." Journal of natural products, 2006; 69(8): 1168-1171.
- Harinantenaina L, Tanaka M, Takaoka S. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem Pharm Bull (Tokyo), 2006, 54, 1017–1021.
- Tan MJ, Ye JM, Turner N. Antidiabetic activities of triterpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol., 2008, 15, 263–273.
- Aswar, PB and Kuchekar, BS. “Photochemical, Microscopic, Antidiabetic, Biochemical and Histopathological Evaluation of Momordica charantia”. International Journal of Pharmacy and Pharmaceutical Sciences, 2012; 4: 325-331.
- Wehash FE, Abpo-Ghanema II, and Saleh RM. “Some physiological effects of Momordica charantia and Trigonella foenum-graecum extract in diabetic rats as compared with Cidophage® World”. Academy of Science, Engineering and Technology, 2012; 64: 1206-1214.
- Virdia J, Sivakamia S, Shahanib S, Sutharc AC, Banavalikar MM, Biyanic MK. Antihyperglycemic effects of three extracts from Momordica charantia. J Ethnopharmacol, 2003, 88:107–111
- Tiwari, P., Mishra, B. N. and Sangwan, N. S. Phytochemical and pharmacological properties of Gymnema Sylvestre: an important medicinal plant, BioMed Research International, 2014, Article ID 830285, 18 pages http://dx.doi.org/10.1155/2014/830285
- Patel, S. S., Shah, R. S. and Goyal, R. K. “Antihyperglycemic, antihyperlipidemic and antioxidant effects of Dihar, a polyherbal ayurvedic formulation in streptozotocin-induced diabetic rats,” Indian Journal of Experimental Biology, 2009, 47 (7): 564– 570,
- Bone, Kerry. Gymnema: a key herb in the management of diabetes.(Phytotherapy Review & Commentary.). Townsend Letter for Doctors and Patients, 2002; 233: 28-31.
- Ishijima, S., Takashima, T., Ikemura, T. and Izutani, Y. “Gymnemic acid interacts with mammalian glycerol-3-phosphate dehydrogenase,” Molecular and Cellular Biochemistry, 2008, 310(1- 2): 203–208
- Sugihara, Y. , Nojima, H., Matsuda, H., Murakami, T., oshikawa, M. Y. and. Kimura, I. “Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema Sylvestre leaves in streptozotocin-diabetic mice,” Journal of Asian natural products Research, 2000, 2(4): 321–327
- Kang MH, Lee MS, Choi MK, Min KS, Shibamoto T. Hypoglycemic activity of Gymnema Sylvestre extract on oxidative stress and antioxidant status in diabetic rats. Journal of Agricultural and Food Chemistry. 2012;60(10):2517-24.
- Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific Journal of Tropical Biomedicine. 2012; 2:320-30.
- Araujo AJS, Jesus-Lima JCR, Otoch JP, and Pessoa AFM. Effect of Ginger (Zingiber officinale) Supplementation on Diabetes: An Update, Am J Phytomed Clin Ther., 2018, l.6.(3):1-20A
- Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem Toxicol, 2008, 46: 409-420.
- Butt, Masood Sadiq, and M. Tauseef Sultan. "Ginger and its health claims: molecular aspects." Critical reviews in food science and nutrition, 2011; 51, (5): 383-393.
- Arablou T, Aryaeian N, Valizadeh M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr, 2014; 65: 515-520.
- Mozaffari KH, Talaei B, Jalali BA. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement Ther. Med., 2014, 22: 9-16.
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Sang, S. Bioactive ginger constituents alleviate protein glycation by trapping methylglyoxal. Chem. Res. Toxicol. 2015, 28, 1842–1849. [CrossRef] [PubMed]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88.
- Bin Samad, M.; Bin Mohsin, M.N.A.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Ul Kabir, A.; Hannan, J.M.A. [6]-Gingerol, from Zingiber o_cinale, potentiates GLP-1 mediated glucose-stimulated insulin secretion pathway in pancreatic beta-cells and increases RAB8/RAB10-regulated membrane presentation of GLUT4 transporters in skeletal muscle to improve hyperglycemia in Lepr(db/db) type 2 diabetic mice. BMC Complem. Altern. M. 2017, 17, 395.
- Li, Y.; Tran, V.H.; Kota, B.P.; Nammi, S.; Duke, C.C.; Roufogalis, B.D. Preventative effect of Zingiber o_cinale on insulin resistance in a high-fat high-carbohydrate diet-fed rat model and its mechanism of action. Basic Clin. Pharmacol. 2014, 115, 209–215. [CrossRef]
- Dongare, S.; Gupta, S.K.; Mathur, R.; Saxena, R.; Mathur, S.; Agarwal, R.; Nag, T.C.; Srivastava, S.; Kumar, P. Zingiber Officinale attenuates retinal microvascular changes in diabetic rats via anti-inflammatory and antiangiogenic mechanisms. Mol. Vis. 2016, 22, 599–609.
- Chakraborty, D., Mukherjee, A., Sikdar, S., Paul, A., Ghosh, S. and Khuda-Bukhsh, A. R. “[6]-Gingerol isolated from ginger attenuates sodium arsenite-induced oxidative stress and plays a corrective role in improving insulin signaling in mice,” Toxicology Letters, 2012, 210(1): 34–43, 2012.
- Heimes, K., Feistel, B. and Verspohl, E. J. “Impact of the 5-HT3 receptor channel system for insulin secretion and interaction of ginger extracts,” European Journal of Pharmacology, 2009, 624(1–3): 58–65
- Putnik, P.; Lorenzo, J.; Barba, F.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursa´c Kovaˇcevi´c, D. Novel food processing and extraction technologies of high-added value compounds from plant materials. Foods 2018, 7, 106. [CrossRef] [PubMed]
- Giacometti, J.; Bursa´c Kovaˇcevi´c, D.; Putnik, P.; Gabri´c, D.; Biluši´c, T.; Kreši´c, G.; Stuli´c, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G. Extraction of bioactive compounds and essential oils from Mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113,245–262. [CrossRef] [PubMed]
- de Bock M, Derraik JGB, Brennan JM. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: a randomized, placebo-controlled, crossover trial. PLoS ONE, 2013, 8 (3), e57622
- Wainstein J, Ganz T, Boaz Ml. Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and rats. J Med Food, 2012, 15, (7) 1-6.
- Temiz MA, Temur A. leaf The effect of olive leaf extract on digestive enzyme inhibition and insulin production in streptozotocin-induced diabetic rats. Ankara Üniv Vet Fak Derg, 2019, 66, 163-169
- Cheng AYY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. Can Med Assoc., 2005, J, 172 (2), 213-226.
- Changrong G, Yingbiao Z, Yiping Y, Riqiu C. Study on the evaluation of the curative effect of berberine in the treatment of type 2 diabetes and its safety. China Modern Doctor. 2017, 33: 82–3384 (In Chinese).
- Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y, Yang D, Liang H, Ye J, Weng J. Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS One 2011 Feb 3;6(2):e16556.
- Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab Jan 2008;294(1): E148-56.
- Lu SS, Yu YL, Zhu HJ. Berberine promotes glucagon-like peptide-1(7-36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol Feb 2009;200(2):159-65.
- Yu Y, Liu L, Wang X, Liu X, Xie L, Wang G. Modulation of glucagon-like peptide-1 release by berberine: in vivo and in vitro studies. Biochem Pharmacol. 2010 Apr 1;79(7):1000-6. DOI: 10.1016/j.bcp.2009.11.017. Epub 2009 Nov 27.
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine a natural plant product activates AMP-activated protein kinase with beneficial metabolic effect in diabetic and insulin-resistant states. Diabetes 2006; 55 (8): 2256-2264.
- Kong WJ, Zhang H, Song DQ. Berberine reduces insulin resistance through protein kinase C-dependent up-regulation of insulin receptor expression. Metabolism. 2009;58(1):109-119
- Jeong HW1, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, Kim WS, Kim JB. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296(4):E955-964
- Ming Zhang, L.C. Berberine in type 2 diabetes therapy: a new perspective for an old antidiarrheal drug?, Acta Pharmaceutica Sinica B 2012;2(4):379–386.
