Archive \ Volume.11 2020 Issue 2
Study on differentiation of pathogen-nonpathogen Mycobacterial infections using ESAT6-CFP10 in ELISA system
Anahita Bahmanjeh 1, Saeed Ataei Kachooei 2*, Mohammad Faezi Ghasemi 1, Nader Mosavari 2, Seyed Mehdi Hassanzadeh 3
1 Department of Microbiology, Faculty of Basic Sciences, Lahijan Branch, Islamic Azad University, Lahijan, Iran. 2 Razi Vaccine and Serum Research Institute, Agricultural Research Education and Extension Organization (AREEO), Tehran, Iran. 3 Vaccine Production Unit, Research & Production Complex, Pasteur Institute of Iran, Karaj, Iran.

Abstract
Pathogenic mycobacteria, including the causative agents of tuberculosis, are responsible for considerable morbidity and mortality worldwide. The availability of a laboratory method that can distinguish between two groups of pathogens; Mycobacterium tuberculosis complex (MTC or MTBC), nontuberculous mycobacteria (NTM) and those who have only had a history of dealing with non-pathogenic or recently vaccinated individuals is of great importance. In this regard, different strains of mycobacteria were cultured in the Dorset-Henley liquid medium and the inactivation of the cultures is carried out by the heat. The bacteria were separated from the liquid medium containing secreted proteins with EKS filters. Low molecular weight proteins in Mycobacterium tuberculosis precipitated with ammonium sulfate were purified by Sephadex-G50 gel chromatography and crude antigens in other mycobacteria precipitated with TCA. Protein concentrations determined with lowry protein assay, antigens coated on the ELISA plate and the results were analyzed with SPSS software and investigated. In this study, all antigens had more than 92% detection ability in healthy livestock in the ELISA method. The highest specificity was related to ESAT-6/CFP10 and M. avium subsp. Paratuberculosis antigens were 83.66% and 95.83% respectively and the highest efficiency of diagnostic tests were over 83% concerning these two antigens. It concluded that the two antigens ESAT-6/CFP10 and M. avium subsp. Paratuberculosis are suitable candidates for the design of the diagnostic ELISA system due to their sensitivity, specificity and efficiency and also reliable detection of healthy livestock from sensitized livestock.
Keywords: Mycobacteria, ELISA, Tuberculosis, livestock

INTRODUCTION
Mycobacterium is a genus of Actinobacteria, given its own family, the Mycobacteriaceae. Mycobacteria can be classified into several major groups for diagnosis and treatment: Mycobacterium tuberculosis complex, which can cause tuberculosis and nontuberculous mycobacteria (NTM) are all the other mycobacteria, which can cause pulmonary disease resembling tuberculosis, lymphadenitis, and skin disease or disseminated disease [1, 2].
Tuberculosis (TB) remains a global health problem, affecting millions of people annually [3]. The main cause of TB is Mycobacterium tuberculosis (MTB), a small, nonmotile, aerobic bacillus [4].
Traditional culture tests are prone to a high rate of false positives due to cross-reactivity with nontuberculous mycobacteria although wider use of liquid cultures are recommended by the World Health Organization (WHO) in countries with high tuberculosis (TB) burden. Thus, it is important to rapidly identify MTB in positive liquid cultures to confirm TB infection [5, 6].
Sensitive and rapid TB diagnostics play an important role in detecting and preventing the disease. Various culture filtrate protein factions have been proposed for the detection of the disease, including MPT64, ESAT-6, CFP-10, Ag85 complex [7, 8]. ESAT6/CFP10 are early-secreted proteins with molecular weights of 6 and 10 kDa, respectively. In culture, they are some of the most abundant MTB antigens. They are not found in Mycobacterium bovis BCG or most Mycobacterium other than tuberculosis (MOTT) but are present in Mycobacterium kansasii, Mycobacterium szulgai, Mycobacterium marinum, and Mycobacterium riyadhense [9].
MPT64 is a secreted, immunogenic protein of MTB. It is highly specific for the Mycobacterium tuberculosis Complex (MTBC), and most importantly; it is not found in MOTT [10].
The Mycobacterium Ag85 complex is involved in biological processes including immunogenic responses and cell wall biosynthesis. For the intracellular survival of MTB within the macrophage, Ag85 expression is essential [11].
ESAT6/CFP10 are also being studied as alternatives to PPD (purified protein derivative). They have shown high sensitivity in the diagnosis of active and new TB infections. Furthermore, no false positives in BCG-vaccinated people have been observed. It has been found that TB proteins actively secreted in culture (Mentioned above) is stronger than that resulting from the standard PPD [12].
For detection and control of bovine tuberculosis (TB), tuberculin test is the most common method used. Due to having low specificity and sensitivity of this tuberculin test, it is better to perform another test in parallel for the investigation of the disease. Recently, due to having the ability of being performed in intermediate-level laboratories with relatively simple equipment, antigen or antibody detection tests such as enzyme immunoassays have largely replaced culture for the diagnosis of many infectious diseases. The ELISA test can be an appropriate test for performing in parallel with the tuberculin test for accurate detection due to bein user-friendly. Several antigens have been evaluated for their serodiagnostic potential in detecting circulating antibodies by enzyme-linked immunosorbent assay (ELISA).
In this study, it was attempted to used ELISA tests based on in cattle for differentiation of the pathogen and non-pathogen mycobacterial infections with proteins smaller than 10 kDa (specially ESAT-6/CFP-10) for the detection of TB and some other antigenic preparations seral antibodies.
MATERIALS AND METHODS
Bacterial species. Four strains of Mycobacterium including Mycobacterium tuberculosis (ATCC: 35808), Mycobacterium phlei (ATCC: 11758), Mycobacterium bovis (ATCC: BCG Pasteur 1173P2), and Mycobacterium avium subsp. Paratuberculosis (ATCC: 316F) were used in this study. All the strains were supplied by the Tuberculin and Mallein Production & Research Department at Razi Vaccine and Serum Research Institute and identifications were confirmed by specific PCR methods previously.
Bacterial culture. The bacteria were subcultured on the Dorset-Henley liquid medium, for 60 days at 37°C to produce a seed culture. After 60 days, Fernbach flask containing medium and bacterium within 60 minutes in the temperature 68° were used for inactivation of live bacteria. The cells were removed from the culture medium by 0.22 µm filters (EKS) filter.
TCA precipitation. The proteins in the culture filtrate of Mycobacterium tuberculosis were mixed thoroughly while one part TCA was added to nine parts culture filtrate resulting in a solution with a final concentration of 4% TCA, described as [13]. This method of precipitation was used for the treatment of 4 strains including Mycobacterium tuberculosis, Mycobacterium phlei, Mycobacterium bovis BCG, and Mycobacterium avium subsp. Paratuberculosis.
Ammonium sulfate precipitation. The proteins in the culture filtrate were precipitated by adding a concentration gradient of ammonium sulfate. The amount of ammonium sulfate that should be added to the solution can be determined by using an online calculator. 561 g ammonium sulfate (http://www.encorbio.com/protocols/AM-SO4.htm) instead of per liter was added from the liquid medium to prepare the solution and then the prepared solution was mixed for 16 h at for 4° C. Then this solution was allowed to stand overnight at room temperature without stirring. The solution was centrifuged in 3,000 rpm for 15 min and its supernatant was discarded, then phosphate buffer, pH:6.8, was added to the residue precipitation. Dialysis against deionized water was used for 72 h to omit the remaining salts in the protein solution, to reassure the exit of salt from the protein solution by conductometer, the conductivity of above solution was studied. After the dialysis process, the contents of the dialysis bag were concentrated with polyethylene glycol, Sigma-Aldrich [14].
Chromatography. Gel chromatography using Sephadex-G50, in a column with a dimension of 150×2 cm, was used for protein fractionation. Phosphate buffer (50 mM, pH 6.8) was added to the concentrated protein solution. Impurities of the protein solution were removed by centrifugation (15 min at 4°C at 10,000 rpm). The supernatant was applied to a gel chromatography column equilibrated previously with 50 mM phosphate buffer (pH 6.8). The column was eluted by 50 mM phosphate buffer (pH 6.8). After chromatography steps for fractionating the obtained proteins, the absorption of protein solutions was read by spectrophotometer in the wavelength 280 nm and its chromatograph was drawn [15].
Protein determination. The amount of yielded proteins in solutions was measured by the Lowry method [16]. In this process, Bovine Serum Albumin (BSA) was used as a standard. The absorbance of samples was read at 750 nm by a spectrophotometer. For protein measurement, Lowry protocol was used as described [16].
SDS-PAGE. Protein electrophoresis was carried out as described by Laemmli [17], using 12% acrylamide/bisacrylamide gels. The proteins were diluted in sample loading buffer, heated to 95°C for 10 min in loading buffer and loaded onto the gel. Silver nitrate and Coomassie Blue R-250 staining were used to detect proteins after electrophoretic separation on polyacrylamide gels [18, 19].
Study populations. Serum samples obtained at different phases of the study from healthy, naturally and infected or experimentally infected cattle with a different antigen of M.tuberculosis, M.phlei, M.bovis, M.avium subsp. paratuberculosis crude antigens. Serum samples were collected from 54 livestock, 37 cows that three continual tuberculin tests were negative and 17 serum samples were positive (in the tuberculin test). Eight of the seventeen sera were sensitized for having positive sera of different strains of mycobacteria at the Tuberculin and Mallein Production & Research Department in Razi Vaccine and Serum Research Institute with killed mycobacteria (crude antigens). Hence 8 Holstein bulls aged 1 to 6 years which had the lowest antibody titers against mycobacterial antigens, were sensitized by using crude antigens of mycobacteria; based on OIE guidelines, crude antigens were mixed with adjuvant and then injected intramuscularly, 1 ml solution, three times with two weeks interval (first injection with Freund's complete adjuvant and next injections with incomplete Freund's adjuvant) and each time before each injection, serum was taken from the cow to investigate antibody titer. All eight livestock were negative for mycobacterial culture before and after two months of sensitization. The other nine positive sera were positive by both the tuberculin test and culture from lymph nodes (or tissues) of tuberculin positive cows.
Tuberculin Skin test. Each dose (0.1 ml) contains 3,250 International Units (IU) Purified Protein Derivatives of Mycobacterium bovis AN5 (PPD). It was provided by the Tuberculin and Mallein Production & Research Department in Razi Vaccine and Serum Research Institute. Before injection, skin thickness was measured using a caliper. 72 hours after injection, the skin of the area was examined, and if any bumps and stiffness were observed at the injection site, its thickness was measured with a caliper and recorded. The thickness is greater than 4 mm in cattle with tuberculosis, between 2 and 4 mm in suspicion, and less than 2 mm in non-infected cattle.
Optimizing the best concentration of mycobacteria, antigens and antibody dilution with Checkerboard and Indirect ELISA test protocol. Due to the optimization of test conditions, separately the checkerboard evaluated antibodies and antigens dilution levels with indirect ELISA method. By adding antigens of different mycobacteria in a separate plate, with concentration range between 1-20 µg/mL, to the bicarbonate coating buffer 0.1 M, pH: 9.6, serial dilution was prepared and 100μl was added in each well of ELISA microtiter plate, PolySorp Nunc-Immuno 96- microwell plate, from column A to G and the last column H, was considered as negative control, coating buffer without antigen. According to the checkerboard, the best serum and antigen dilutions were identified. The microtiter plate was incubated for 16 to 24 hours at 4°C. Then, the wells were washed three times with 300 μl of 10 mM PBS solution and then each well was filled with 150μl of PBS Tween20 and solution of 2.5% casein (Maravel -UK) as a blocker and incubated for one hour at room temperature. The obtained dilutions of 1/50 and 1/100 negative and positive control serum that were prepared by using 10 mM PBS/Tween 20 with BSA 1%, was added to each well with the volume of 100 μl and were incubated for 30 minutes at room temperature, 20 to 25°C. After 5 times washing with 300 μL of PBST, 10 mM PBS, containing 0.1% Tween 20, optimized dilution of 1/5000 was prepared in 10mM PBS, pH 7.2, from HRP conjugate, Anti-Bovine Anti-IgG Horseradish Peroxidase Conjugated Antibody, AbD Serotec-UK, and 100 μl was added to each well and the plate was incubated again for 30 min at room temperature. Subsequently, after washing wells, according to the previous stage, 100 microliters of 3,3′,5,5′-Tetramethylbenzidine or TMB substrate were added to wells and the plate maintained for 15 minutes in darkness at room temperature. Finally, 100 μl of a stop solution, H2SO4 0.1M, was added and the plate was read at a wavelength of 450 nm by an ELISA reader, Bio Rad-Model 620. Blocker buffers used in the optimization process include the PBS Tween20 and solution of 2.5% casein (Maravel -UK).
Sensitivity and specificity of the ELISA test. Sensitivity and Specificity were determined by formula “Sensitivity= true positives/ (true positive + false negative)” and “Specificity= true negatives/ (true negative + false positives)” based on new S/P.
Data Analysis. The cutoff value for the ELISA assay was calculated using the ROC curve based on 54 serums with 37 negative PPD skin tests and 17 positive PPD skin as a standard test. Statistical analyses were performed with the SPSS software version (25.0.0.0).
RESULTS
Ammonium sulfate precipitation. Since it is possible to separate proteins with molecular weights below 30 kDa by ammonium sulfate precipitation, for isolation of ESAT-6/CFP-10 at a molecular weight of 14 kDa from the M. tuberculosis crude antigens, ammonium sulfate precipitation was used. After dialysis, the conductometer showed a number below 1000 rpm, indicating salt was removed from the solution.
Chromatography for protein determination. To isolate different fractions of the proteins present in the crude antigens of M.tuberculosis, gel filtration was used. After separation of fractions and drawing a chromatograph, three peaks were observed (Fig. 1) and studying the protein concentration in each fractions with Lowry protein assay determined that out of, 8.65 mg/ml protein in fraction 1, 3.56 mg/ml protein in fraction 2, 0.6 mg/ml protein in fraction 3 proteins were the proteins from high to low molecular weight. (Fig. 1).
SDS-PAGE of fraction-2 antigen. It was observed that fraction 2 (F2) has a two-band containing the proteins with low molecular weight 10 and 14.4 kD (Fig. 2), using molecular weight assay by SDS-PAGE and staining with silver nitrate. The molecular weights of protein from M. tuberculosis ranged from 6 to 180 kDa at this stage. The ESAT6/CFP10 protein complex is one of the most important M. tuberculosis secreted proteins with molecular weight 14 kD (Fig. 2). All processes were performed on fraction 2.
TCA precipitation for protein determination. The TCA precipitated the method with different concentrations of proteins of both high molecular weight and low molecular weight (total proteins). The concentration of M. bovis BCG, M. phlei, M. avium subsp. Paratuberculosis and M. tuberculosis proteins precipitation TCA were estimated by Lowry protein assay at 1.5 mg/ml, 5.65 mg/ml, 1.8 mg/ml and 1.5 mg/ml, respectively.
SDS-PAGE of different mycobacterial crude antigens. Crude antigens isolated by TCA were loaded onto a 12% separator gel with 10 to 180 kDa Protein Markers to evaluate the weights and the presence of proteins in different mycobacteria. After deposition of the samples and staining with Coomassie Blue method, the presence of proteins with different molecular weights was determined. SDS-PAGE proteins of M. tuberculosis, M. avium subsp. Paratuberculosis, M. phlei, M. bovis BCG are shown in (Fig. 3). As a result, the protein accumulation occurs at different molecular weights with 35 to 180 kDa, the number of bands observed in each Mycobacterium preparation is about 15 to 17 bands.
Checkerboard results in the ELISA method. The results of the Checkerboard showed that the best-selected concentrations of M. bovis BCG, M. phlei, M. avium subsp. Paratuberculosis and M. tuberculosis, ESAT6/CFP10 antigens after optimization of test conditions to increase a ratio (S/N) were 7.5 µg/ml, 15 µg/ml, 0.9 µg/ml, 7.5 µg/ml, and 7.5 µg/ml, respectively; and the appropriate dilution used for 54 sera, positive and negative control was 1/50.
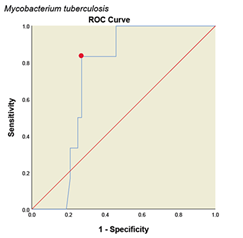
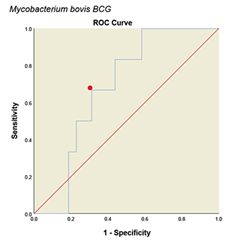
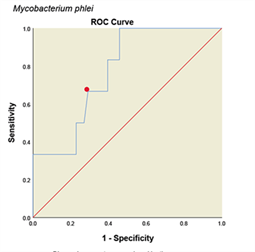
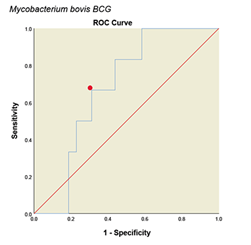
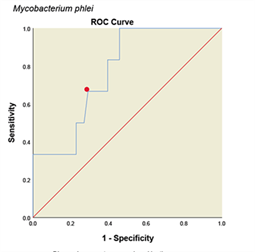
Determining Cutoff for ELISA system with ROC curve. ROC curve is a graph or graphical schema that uses the true positive rate (sensitivity) on the x-axis and the false positive rate (specificity-1) on the Y-axis at different thresholds (different cutoff points) is created. All analyses and graphs are drawn with SPSS software version (25.0.0.0) and based on the above data the ROC curves for the method are shown in (Fig. 4). The areas under the curves with 95% CIs and with cut off value were calculated in (Table 1). Accordingly, the optimal cutoff values of the optical densities of antibody responses to the ESAT-6/CFP10, M. tuberculosis, M. phlei, M. avium subsp. Paratuberculosis, M. bovis BCG antigens for diagnosing livestock contaminated with these mycobacteria were 0.921, 0.452, 0.257, 0.436 and 0.923, respectively (Table 1).
Determining the sensitivity and specificity of the ELISA absorption system to compare with skin test results. Statistical indices of sensitivity, specificity, positive and negative predictive value were calculated for data analysis. Test sensitivity for ESAT-6/CFP10, M. tuberculosis, M. avium subsp. Paratuberculosis, M. phlei, M. bovis BCG antigens is 60%, 83.33%, 33.33%, 66.66%, 66.66% respectively, having specificity, 88.63%, 72.91%, 95.83%, 70.83%, 68.75% , respectively. Efficiency of test is 83.33%, 74.07%, 88.88%, 70.37%, and 68.51%, respectively (Table 2).
Agreement of the tests based on Kappa coefficient calculation
Based on the PPD skin test as the standard, other tests were compared with this test. In ESAT6/CFP10 antigen, M. tuberculosis, M. avium subsp. Paratuberculosis, M. phlei and M. bovis BCG with PPD skin test, Kappa agreement coefficient is 0.468 (P<0.01), 0.3 (P<0.01), 0.341 (P<0.05), 0.2 (P<0.05) and 0.182 (P<0.05), respectively.
DISCUSSION
Tuberculosis remains a global health problem. In 2017, there were an estimated about 11 million new cases and 1.4 million deaths due to TB, worldwide [20]. The prompt diagnosis of mycobacteria especially MTB is a crucial problem [21]. Although the golden standard for diagnosing active TB remains the culture of the causative organism, but takes a long time and is not always successful. The serological method is an effective diagnostic tool of TB in vitro, which would be an attractive progress as immunoassays are simple, rapid, inexpensive and may offer the possibility to detect cases missed by standard sputum smear microscopy [21].
In this study, the results of the SDS-PAGE method of precipitation with TCA have almost the same band pattern because of the isolated protein complex in mycobacteria having the same protein profile. Ultimately, the SDS-PAGE method revealed that the ammonium sulfate precipitate was better than the precipitate with TCA because nonspecific bands were rarely observed. Three fractions obtained by chromatography, fraction-2 of M. tuberculosis antigen were more suitable than other fractions because they had appropriate molecular weight and acceptable ELISA results, and fraction-2 of M. tuberculosis antigen was used in the ELISA test.
There is a Kappa coefficient between ESAT6/CFP10 antigen with PPD skin test 0.468 and P> 0.05, this test can be a good alternative to the PPD test. Notable P> 0.05 indicates that the results are not random.
ESAT6/CFP10 antigens with a sensitivity of 60% and a specificity of 88.63% indicate the ability of the test to acceptably differentiate non-diseased livestock. These antigens are a suitable candidate for the design of the diagnostic ELISA system with a test efficiency of 88.33%. The negative predictive value of this experiment was 90.69%, indicating a good ability to identify healthy livestock and positive predictive value was 54.54%.
Given the false-negative results of the ELISA test, there may be a recent infection with M. tuberculosis that has been treated or despite M. tuberculosis in livestock, the PPD skin test is unable to detect active disease states or memory antibodies in livestock serum. It was also shown by our previous study in 2014 that performing the ELISA test based on proteins lower than 10 kDa M.W. like ESAT-6 antigen isolated from sonicated bacteria was precipitated with ammonium sulfate, that can be a suitable way for screening besides the tuberculin test for accurate detection [22].
In NTM mycobacteria like M. avium subsp. Paratuberculosis antigens, there is a kappa coefficient between M. avium subsp. Paratuberculosis with PPD skin testes 0.341 and P> 0.05, so the agreement between the two tests is low and this test cannot replace PPD skin test. With a sensitivity of 33.33% and a specificity of 95.83%, this test is capable of detecting healthy livestock; the test efficiency is 88.88%. Given the above feature, it is possible to design an ELISA system for the diagnosis of M. avium subsp. Paratuberculosis. Negative predictive 92%, indicates a good ability to detect healthy livestock and positive predictive value of 50%, indicates good testability in the detection of M. avium subsp. Paratuberculosis cases. In one of our previous studies in 2016, specificity and sensitivity of the system were determined by 99% and 86%, respectively. These results showed that secreted antigens of Mycobacterium avium subsp. Paratuberculosis can be one of the top choices for developing new ELISA systems because the most present kits are cellular antigens. Compared to the method performed, to remove non-specific antibodies in the bovine serum, soluble antigens of M. phlei were used [23].
In M. phlei antigens, there is a kappa coefficient between M. phlie with PPD skin test 0.2% and P> 0.05, so the agreement between the two tests is not suitable and this test cannot replace PPD skin test. This test with 66.66% sensitivity, 70.83% specificity and 70.37% efficiency can detect healthy livestock but the ability of the test to correctly detect sick livestock is mediocre. There is a low possibility with this method to design an ELISA system for detection of M. phlie. The negative predictive value was 94.44% which indicates a good ability to detect healthy livestock and 22% positive predictive value indicating very low ability in detecting livestock infected with M. phlie, because of the high antigenic crosses observed in the diagnosis of M. phlie in this study.
In M. bovis BCG antigens, there is a Kappa coefficient between M. bovis BCG with PPD skin test 0.182 and P> 0.05 between M. bovis BCG with PPD skin test, so the agreement between two tests is not appropriate and this test cannot replace PPD. This test, with a sensitivity of 66.66% and a specificity of 68.75% has an average ability to detect healthy and infected livestock. The test efficiency is 68.51%. It is impossible with this system to design an ELISA system for the detection of M. bovis BCG. Negative predictive value 94.28, indicates a good ability to detect healthy livestock and positive predictive value 21.05% indicating the very low ability of testing due to high cross-antigenic is detectable in M. bovis BCG cases. As a result, cattle recent immunization with the BCG vaccine, cannot be detected by ELISA system. High antigenic crosses were present in the detection of BCG and M. phlei -infected sera in this research.
In M. tuberculosis antigens, there is a Kappa coefficient between M. tuberculosis with PPD skin test 0.30 and P> 0.05 between M. tuberculosis with PPD skin test, so the agreement between the two tests is low and this test cannot replace PPD skin test. The sensitivity of the ELISA test is 83.33% specificity of the test is 72.91% and the efficiency of the test is 74.04% . This antigen is a suitable candidate for the design of the diagnostic ELISA system. The negative predictive value of this test was 97.22% indicating a good ability to detect healthy livestock. The positive predictive value of 27.77 indicates a low test ability to detect cases of M. tuberculosis and the presence of the antigenic cross. The report about the use of M. tuberculosis-secreted antigens, that shows these antigens have a sensitivity of 67% and a specificity of 79% for patients with M. tuberculosis with ELISA, dates back to 1984 [24]. Higher sensitivity was observed in the present study compared with the Zeiss et al. study.
It is concluded from the present study that the two antigens ESAT-6/CFP10 and M. avium subsp. Paratuberculosis crude antigens are proper candidates for the design of diagnostic ELISA due to their sensitivity, specificity, and efficiency. The highest sensitivity of ELISA assays was in M. tuberculosis antigen which was 83.33%. The highest specificity was related to ESAT-6/CFP10 and M. avium subsp. Paratuberculosis antigens which were 83.66% and 95.83%, respectively and the highest efficiency of diagnostic tests were over 83% concerning these two antigens. The actual negative results in ESAT-6/CFP10 antigen and M. avium subsp. Paratuberculosis antigens had more than 90% detection rate in healthy livestock. M. avium subsp. Paratuberculosis was well diagnosed in two crude antigen-sensitive livestock of M. avium subsp. Paratuberculosis. All antigens studied had more than 90% detection ability in healthy livestock.
This study showed that precipitating M. tuberculosis antigens with ammonium sulfate salts compared with TCA precipitating, had higher specificity and positive predictive value. The many different protein fractions present in the whole-protein antigen extract may explain its greater reactivity. It is recommended that one or more specific or recombinant antigens be used to obtain better results in the design diagnostic ELISA system or to remove non-specific antibodies in the bovine serum, soluble antigens of M. phlie can be used [23].
More studies are required in the format of field trials as a proof for ELISA systems developed by the present study.
ACKNOWLEDGMENTS
We are thankful to all personnel of Razi Vaccine and Serum Research Institute especially Tuberculin and Mallein Production & Research Department, Vaccine Production Unit, Research & Production Complex, and also Arna Gene Avisa Company that provided support.
Conflict of Interest
The authors declare no conflict of interest.
Authors’ Contributions
Conceptualization and methodology: Nader Mosavari and Saeed Ataei Kachooei
The investigation, data curation and original draft preparation: Anahita Bahmanjeh
Review and editing: Saeed Ataei Kachooei, Anahita Bahmanjeh and Nader Mosavari
Software and Formal analysis, review: Mohammad Faezi Ghasemi
Validation, review: Seyed Mehdi Hassanzadeh.
REFERENCES
- Tortoli E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clinical microbiology reviews. 2014;27(4):727-52.
- Tortoli E, Fedrizzi T, Meehan CJ, Trovato A, Grottola A, Giacobazzi E, Serpini GF, Tagliazucchi S, Fabio A, Bettua C, et al. The new phylogeny of the genus Mycobacterium: the old and the news. Infection, Genetics and Evolution. 2017;56:19-25.
- Zaman K. Tuberculosis: a global health problem. Journal of health, population, and nutrition. 2010;28(2):111.
- Dunn JJ, Starke JR, Revell PA. Laboratory diagnosis of Mycobacterium tuberculosis infection and disease in children. Journal of clinical microbiology. 2016;54(6):1434-41.
- Parsons LM, Somoskövi Á, Gutierrez C, Lee E, Paramasivan C, Abimiku Al, Spector S, Roscigno G, Nkengasong J. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clinical microbiology reviews. 2011;24(2):314-50.
- Lawn SD. Advances in diagnostic assays for tuberculosis. Cold Spring Harbor perspectives in medicine. 2015;5(12):a01780
- Bai Y, Xue Y, Gao H, Wang L, Ding T, Bai W, Fan A, Zhang J, An Q, Xu Z. Expression and purification of Mycobacterium tuberculosis ESAT-6 and MPT64 fusion protein and its immunoprophylactic potential in mouse model. Protein expression and purification. 2008;59(2):189-96.
- Bekmurzayeva A, Sypabekova M, Kanayeva D. Tuberculosis diagnosis using immunodominant, secreted antigens of Mycobacterium tuberculosis. Tuberculosis. 2013;93(4):381-
- Mahmoudi S, Mamishi S, Ghazi M, Sadeghi RH, Pourakbari B. Cloning, expression and purification of Mycobacterium tuberculosis ESAT-6 and CFP-10 antigens. Iranian journal of microbiology. 2013;5(4):374.
- Arora J, Kumar G, Verma AK, Bhalla M, Sarin R, Myneedu VP. Utility of MPT64 antigen detection for rapid confirmation of Mycobacterium tuberculosis complex. Journal of global infectious diseases. 2015;7(2):66.
- Babaki MKZ, Soleimanpour S, Rezaee SA. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microbial pathogenesis. 2017;112:20-9.
- Van Pinxteren LA, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Diagn Lab Immunol. 2000;7(2):155-60.
- Steadham E, Martin B, Thoen C. Production of a Mycobacterium avium ssp. paratuberculosis purified protein derivative (PPD) and evaluation of potency in guinea pigs. Biologicals. 2002;30(2):93-5.
- Wingfield P. Protein precipitation using ammonium sulfate. Current protocols in protein science. 1998;13(1):A. 3F. 1-A. 3F. 8.
- Sadeghi D, Mosavari N, Rafiee B, Mohamad-Taheri M, Dashtipour S, Zare A, Tebyanian M, Rabii H. Isolation and Purification of Low Molecular Weight Proteins from Liquid Culture for Mycobacterium Tuberculosis by Chromatography. Zahedan Journal of Research in Medical Sciences. 2013;15(5):35-8.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of biological chemistry. 1951;193:265-75.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. nature. 1970;227(5259):680.
- Arndt C, Koristka S, Feldmann A, Bartsch H, Bachmann M. Coomassie-Brilliant Blue staining of polyacrylamide gels. Protein Electrophoresis: Springer; 2012. p. 465-9.
- Chevallet M, Luche S, Rabilloud T. Silver staining of proteins in polyacrylamide gels. Nature protocols. 2006;1(4):1852.
- Sakamoto H, Lee S, Ishizuka A, Hinoshita E, Hori H, Ishibashi N, Komada K, Norizuki M, Katsuma Y, Akashi H, et al. Challenges and opportunities for eliminating tuberculosis–leveraging political momentum of the UN high-level meeting on tuberculosis. BMC public health. 2019;19(1):76.
- Zhang C, Song X, Zhao Y, Zhang H, Zhao S, Mao F, Bai B, Wu S, Shi C. Mycobacterium tuberculosis secreted proteins as potential biomarkers for the diagnosis of active tuberculosis and latent tuberculosis infection. Journal of clinical laboratory analysis. 2015;29(5):375-82.
- Keshavarz R, Mosavari N, Tadayon K, Fardid F, Ghaderi R, Babadi KS, Arefpajoohi R, Sajadi SH, Taheri MM, Dashtipour S, et al. Development and optimization of an ESAT6-ELISA-based detection system of Mycobacterium tuberculosis complex, suitable for bovine TB eradication. International Journal of Mycobacteriology. 2015;4:114.
- Rouhollah K, Ali M, Nader M, Keyvan T, Shamseddin G, Farzaneh F, Shojaat DP. Development and optimization a high sensitive and specific ELISA system for rapid detection of paratuberculosis in cattle. International Journal of Advanced Biotechnology and Research. 2016;7(1):1-9.
- Zeiss C, Kalish S, Erlich K, Levitz D, Metzger E, Radin R, Phair JP. IgG antibody to purified protein derivative by enzyme-linked immunosorbent assay in the diagnosis of pulmonary tuberculosis. American Review of Respiratory Disease. 1984;130(5):845-8.
|
Table 1. The cutoff value was calculated for different mycobacteria |
||||||
|
Test Result Variable(s) OD |
cut off value |
Area |
Std. Errora |
Asymptotic Sig.b |
Asymptotic 95% Confidence Interval(CIs) |
|
|
|
|
|
|
|
Upper Bound |
Lower Bound |
|
ESAT-6/CFP10 |
0.921 |
0.751 |
0.075 |
0.014 |
0.605 |
0.897 |
|
M. tuberculosis |
0.452 |
0.724 |
0.068 |
0.076 |
0.590 |
0.858 |
|
M. phlei |
0.257 |
0.773 |
0.084 |
0.031 |
0.608 |
0.937 |
|
M. avium subsp. Paratuberculosis |
0.436 |
0.616 |
0.117 |
0.356 |
0.387 |
0.846 |
|
M. bovis BCG |
0.923 |
0.677 |
0.081 |
0.160 |
0.517 |
0.837 |
The test result variable(s): ESAT-6-OD, M. tuberculosis, M. avium subsp. Paratuberculosis, M. phlei, M. bovis BCG has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
a. Under the nonparametric assumption
b. Null hypothesis: true area = 0.5
|
Table 2. Determining of sensitivity, specificity, and efficiency of different Mycobacterium |
|||||
|
sensitivity and specificity |
ESAT-6/CFP10 Antigen |
M. tuberculosis |
M. avium subsp. Paratuberculosis |
M. phlei |
M. bovis BCG |
|
Sensitivity (%) |
60 |
83.33 |
33.33 |
66.66 |
66.66 |
|
Specificity (%) |
88.63 |
72.91 |
95.83 |
70.83 |
68.75 |
|
Positive predictive value (%) |
54.54 |
27.77 |
50 |
22.22 |
21.05 |
|
Negative predictive value (%) |
90.69 |
97.22 |
92 |
94.44 |
94.28 |
|
Efficiency (%) |
83.33 |
74.07 |
88.88 |
70.37 |
68.51 |
Determination of sensitivity and specificity, positive and negative predictive value, and the efficiency of the studied livestock based on true positive and true negative and false-positive and false-negative results.
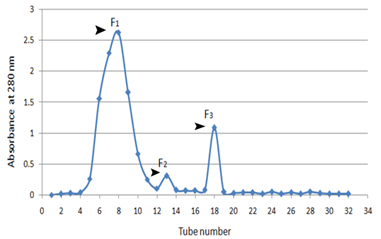
Figure 1. Gel filtration chromatography on crude Mycobacterium tuberculosis antigens
Sephadex G-50 chromatography of M.tuberculosis; Three peaks (F1, F2, and F3) of optical density obtained by chromatography were observed. The first peak consisted of high molecular weight proteins and the second with a medium molecular weight and third peaks contained low molecular weight proteins.
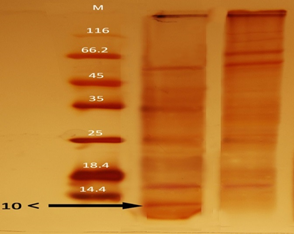
Figure 2. Staining of SDS-PAGE gel with silver nitrate in low molecular weight proteins from M. tuberculosis and the effect of heat and centrifugation on proteins
SDS-PAGE of M. tuberculosis proteins. Two bands are seen in the region of 10 and 14.4 kDa molecular weight, which is in association with the ESAT-6/CFP10.M: Protein marker with molecular weights of 10 to 180 kDa.
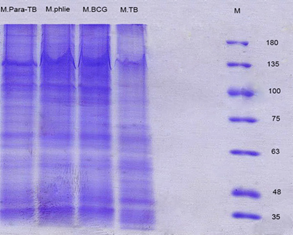
Figure 3. SDS- PAGE gel staining with coomassie blue in different mycobacterial crude antigens
SDS-PAGE of TCA precipitated Proteins, right to left, 10 to 180 kDa protein ladder, M. tuberculosis (M.TB) , M. bovis BCG (M.BCG), M. phlei, M. avium subsp. Paratuberculosis (M.Para-TB), respectively. The number of bands observed in each Mycobacterium preparation is about 15-17 bands. The protein accumulation occurs at different molecular weights.
 |
|
|
(a) |
(b) |
|
|
|
|
(c) |
(d) |
|
|
|
|
(e) |
|
Figure 4: ROC diagram plotted for different Mycobacterium antigens
ROC diagram plotted for different mycobacterial antigens. a: ESAT6/CFP10 antigen, b: M. tuberculosis, c: M. avium subsp. Paratuberculosis, d: M. phlei, e: M. bovis BCG. The PPD skin test was considered as the standard. The red dot on each graph represents the cut point desired based on the sensitivity and specificity.