Archive \ Volume.15 2024 Issue 1
Impact of Carrageenan-Soy Protein Combination on CXCR-4 Expression, Cell Viability, and Apoptosis in HCT-116 Cells
Abstract
The objective was to explore the therapeutic potential of carrageenan and soy protein in treating colorectal cancer (CRC) by selectively targeting cancer cells and modulating the chemokine receptor CXCR-4. The chemokine receptor CXCR-4 plays a crucial role in colon cancer by promoting tumor cell proliferation, metastasis, and angiogenesis. Therefore, targeting CXCR-4 expression could be a promising approach for colon cancer treatment. We conducted experiments using two groups of HCT-116 cells. The CS-HCT group was treated with a combination of carrageenan and soy protein, while the untreated Group UHCT served as a control. The results demonstrated that the combination treatment with carrageenan and soy protein led to a time-dependent decrease in cell viability compared to the untreated group. The treated group exhibited significantly reduced cell viability, particularly after 48 and 72 hours of treatment. Moreover, the combination treatment induced programmed cell death, as evidenced by increased levels of apoptosis after 48 hours. Interestingly, the expression of CXCR-4 was significantly upregulated in response to the carrageenan/soy protein treatment. However, this increase in CXCR-4 expression was associated with elevated apoptosis levels and reduced cell proliferation. Both apoptosis and cell proliferation were enhanced when CXCR-4 expression levels decreased after 48 and 72 hours. Notably, the highest expression of CXCR-4 was observed at 24 hours of treatment compared to later time points. The results underscore the therapeutic potential of carrageenan and soy protein in colon cancer by targeting CXCR-4 expression, requiring further research for comprehensive understanding.
How to cite:
Download Citation
INTRODUCTION
Chemokines are important cytokines that regulate cellular functions [1]. They can be divided into CXC and CC subgroups based on cysteine residues [2]. CXCR-4, a crucial chemokine receptor, binds to CXCL12 and triggers signaling events for various cellular processes [2]. CXCR-4 facilitates the migration of stem cells from the bone marrow to tissues for tissue repair [1]. Elevated CXCR-4 levels contribute to tumor progression by altering cell behavior and promoting survival, angiogenesis, and metastasis in cancer. The CXCR-4/CXCL12 axis promotes tumor formation by directly activating signaling pathways or recruiting CXCR-4/CXCL12-positive cancer cells [3]. The binding of CXCR-4 and CXCL12 activates G proteins, activating downstream pathways, including RAS/RAF, P13K/Akt/mTOR, and MEK/ERK. This activation has anti-apoptotic effects on tumor cells by accumulating NF-κB through ERK and AKT activation. Additionally, active ERK phosphorylates Bim, causing it to dissociate from Bcl-2 and block BAX [4].
Marine organisms produce natural compounds with the potential to treat diseases, including cancer [5]. Polysaccharides from seaweed have anticoagulant, antioxidant, antitumor, and immunomodulatory effects [6]. Over 3000 anti-cancer compounds have been identified from marine sources, with inhibitory effects on cancer cells [6]. Marine polysaccharides enhance immune function and regulate cell cycles, apoptosis-related genes, and signaling pathways [7]. Some seaweed-derived compounds are already used as cancer drugs [8]. Marine natural products offer a promising avenue for developing effective cancer therapies [9].
Soybean contains compounds that can prevent and inhibit cancer growth [10]. These compounds, including phytosterols, phytates, saponins, protease inhibitors, phenolic acids, and isoflavones [11], have hormonal and non-hormonal properties that reduce the risk of various types of cancer [12]. Soy protein isolate has been associated with a decreased occurrence of colon, breast, and prostate cancer [13]. Soybean bioactive peptides, such as isoflavones and genistein [14], can inhibit cancer cell proliferation and induce apoptosis [15]. Genistein is a powerful inhibitor that promotes cell death, inhibits growth, regulates cell cycles, and prevents angiogenesis [16]. It also blocks cancer-stimulating enzymes, reduces hormone levels, and controls tumor angiogenesis [15].
Colorectal cancer (CRC) is a highly prevalent and lethal form of cancer, characterized by the accumulation of genetic and epigenetic alterations over time [17]. Tumor suppressor genes are blocked, and oncogenes are activated in CRC [18]. An unhealthy diet and lifestyle contribute to the high incidence of CRC [19], particularly in countries like Saudi Arabia, Oman, and the UAE [18]. While chemotherapy is commonly used for CRC treatment, it often leads to severe side effects and some patients may develop resistance to chemotherapy [17].
Therefore, considering the importance of investigating natural therapeutic agents for CRC, this study aimed to examine the impact of a mixture of carrageenan and soy protein on the expression of CXCR-4 in the HCT-116 colon cancer cell line at different time intervals. Additionally, the study aimed to evaluate the effects of this treatment on the levels of apoptosis in HCT-116 cells.
MATERIALS AND METHODS
Procurement and Dilution of κ-Carrageenan Sulfated Plant Polysaccharide and Soy Protein for Cell Culture Application
The κ-Carrageenan sulfated plant polysaccharide (powder) was obtained from Sigma-Aldrich with the CAS Catalog Number 11114-20-8, while the soy protein was purchased from iHerp®. Both chemical compounds were diluted and filtered using a 0.22-micron filter. The concentrations were adjusted to approximately 0.025 mg for carrageenan and 0.005 mg for soy protein, which were dissolved in 100 μl of DMEM culture media per 5000 HCT cells.
Culture and Treatment of HCT-116 Colon Cancer Cell Line for Comparative Study
We used HCT-116 (ATCCÒCCL247™) colon cancer cells cultured in DMEM with fetal bovine serum and penicillin/streptomycin. They were kept in a humidified incubator and regularly split when they reached 70% confluency. We assessed their viability using Trypan blue dye [20, 21]. The cells were divided into two groups: untreated (UHC group) and treated with a Carrageenan and Soy protein (CS-HCT group) mixture. Samples were collected after 24, 48, and 72 hours.
Assessment of Cytotoxic Effects on HCT-116 Cells Using MTT Assay
This study examined the effects of different treatments on HCT-116 cells using the MTT assay. Cells were seeded in 96-well plates at a concentration of 5x103 cells/well and treated with either carrageenan/soy protein or left untreated. After incubation, fresh culture medium and MTT solution were added to each well, and the plate was incubated for 4 hours. The formazan crystals were dissolved with DMSO, and the absorbance was measured at 540 nm using a microplate reader [21-23].
Flow Cytometry Analysis of HCT-116 Cell Apoptosis Following Treatment
HCT-116 cells were plated in T-25 flasks (2×106 cells/flask) and allowed to adhere for 24 hours. Treatments were applied for 5 minutes, and the cells were incubated for different durations (24, 48, and 72 hours) before being harvested and washed. They were then transferred to FACS tubes and 5 µl of FITC Annexin V Apoptosis detection kit II (Cat No 51-6710AK) and 5 µl of propidium iodide (PI) were added and analyzed using flow cytometry.
RNA Extraction and Gene Expression Analysis in HCT-116 Cells Following Treatment
HCT-116 cells were cultured in T-75 flasks till the count reached 6x106 per flask, subjected to specific conditions, and collected as pellets after each incubation period. Pellets were stored at -80°C for RNA extraction using the QIAGEN RNeasy® Midi Kit. cDNA was synthesized from RNA with the ImProm-II™ Reverse Transcription System and stored at -20°C. Gene expression analysis was performed by amplifying target-specific genes using BioFact™ 2X Real-time PCR Master Mix [24, 25] and normalizing the expression of GAPDH with data analysis using the 2−ΔΔCt method [26].
Statistical Analysis of HCT-116 Cell Findings
The findings obtained from the study were subjected to statistical analysis using MegaStat® software (Version 10.2 Release 2.1). To assess the statistical significance of variations within the untreated control group and the differences between the treated and untreated groups, a one-way ANOVA (analysis of variance) was employed. A significance level of P-value < 0.05 was used to determine the statistical significance.
RESULTS AND DISCUSSION
Impact of Target Mixture on HCT-116 Cells after 24 Hours of Treatment
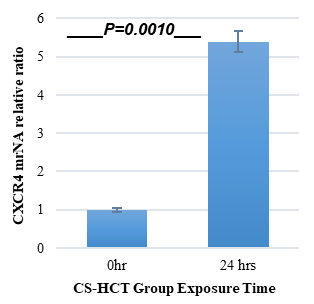
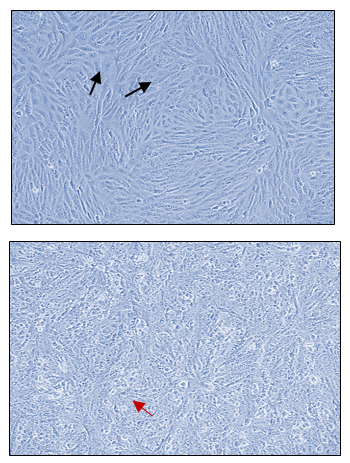
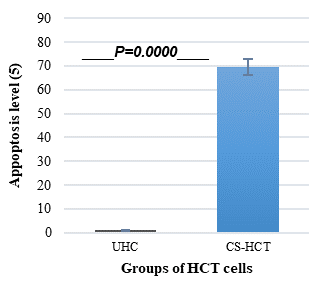
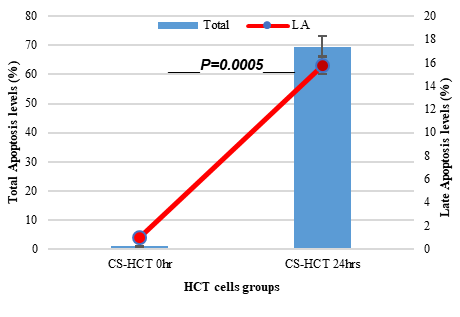
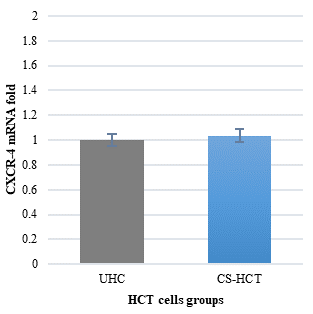
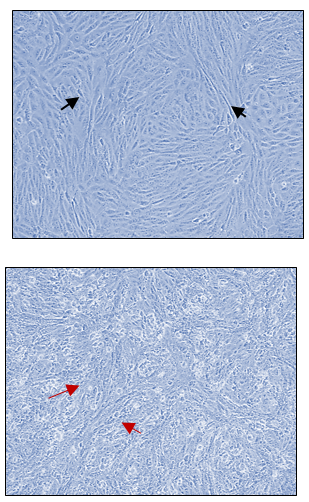
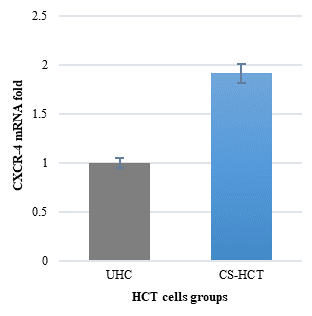
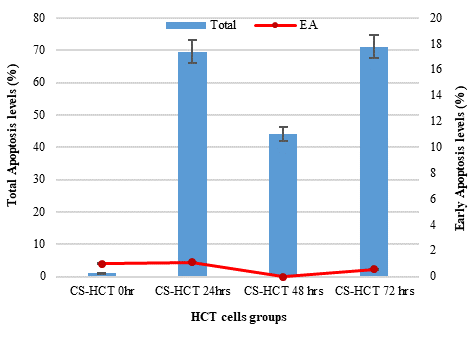
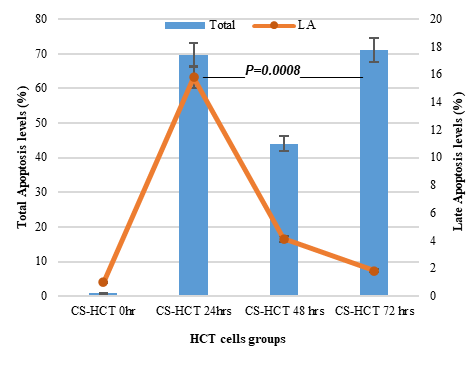
In Figures 1a and 1b, the group of HCT cells treated with a combination of carrageenan and soy protein (CS-HCT) showed a significant increase in the expression of CXCR-4 compared to the untreated control group (p-value = 0.0001), and the group treated for 0 hours (p-value = 0.0001). This increase is visually depicted. Additionally, when HCT cells were treated with a mixture of carrageenan and soy protein, there was a reduction in cell shrinkage, membrane blebbing, and cell fragmentation, although these changes were observed in a smaller subset of cells compared to normal HCT cells (Figure 1c). After 24 hours, the use of carrageenan and soy protein resulted in a significant decrease in cell viability, with a viability percentage of 78.8% compared to the initial cell viability. This decrease in cell viability was statistically significant (p-value= 0.0004) (Figure 1d). Furthermore, the CS-HCT group showed a notable increase in overall apoptosis of HCT-116 cells after 24 hours compared to the untreated control group (p-value = 0.0000), as shown in Figure 1e. Although the combination treatment exhibited a non-significant increase in early apoptosis levels in HCT cells after 24 hours, there was a significant rise in late apoptosis compared to the 0-hour time point (p-value = 0.0005), as indicated by the data presented in Figures 1f and 1g.
Impact of Target Mixture on HCT-116 Cells after 48 Hours of Treatment
After a 48-hour treatment period, the group receiving a combination of carrageenan and soy protein exhibited comparable levels of CXCR-4 expression to both the untreated control group and the 0-hour treatment group (Figures 1a and 1b). However, when compared to the 24-hour treatment group, the carrageenan/soy protein-treated group showed a significant decrease in CXCR-4 gene expression (p-value = 0.0000) (Figure 1b). Morphologically, the HCT-116 cells treated with the carrageenan/soy protein mixture for 48 hours displayed enhanced apoptotic characteristics, such as cell shrinkage, membrane blebbing, cell fragmentation, and condensation and fragmentation of the nucleus, as shown in Figure 1c. Although there was a slight non-significant increase in cell viability after 48 hours in the carrageenan/soy protein-treated group compared to 24 hours, it remained significantly lower than the viability of untreated cells, with a viability percentage of 82.95% (Figure 1d). Statistical analysis confirmed the significance of these changes (p-value = 0.028). Furthermore, there was a marked increase in total apoptosis of HCT-116 cells after 48 hours of treatment with the carrageenan and soy protein mixture compared to both the baseline of the untreated control group and the 24-hour treatment period (p-value = 0.0000 for each time point) (Figure 1e). In contrast, the combination treatment showed a significant decrease in early apoptosis levels in HCT cells compared to levels observed after 0 hours or 24 hours of treatment (p-value=0.0039 and 0.0020, respectively) (Figure 2f). Levels of late apoptosis after 48 hours of exposure to the mixture of carrageenan and soy protein showed an extremely significant decrease compared to the level after 24 hours of treatment (p-value = 0.0031). However, there was a non-significant increase compared to the 0-hour time point, as indicated by the data presented in Figures 2f and 2g.
Impact of Target Mixture on HCT-116 Cells after 72 Hours of Treatment
The HCT cells treated with the carrageenan/soy protein mixture did not show a significant increase in CXCR4 mRNA compared to the untreated control group (Figure 3a). However, there was a significant decrease in CXCR-4 gene expression compared to the 24-hour time point (p-value = 0.0000), but no significant change compared to the 0-hour and 48-hour treatments (Figure 3b). The morphological changes observed in HCT-116 cells after 72 hours of treatment with the carrageenan and soy protein mixture were consistent with the apoptotic features observed at the 48-hour mark (Figure 3c). Although there was a continued increase in cell viability after 72 hours in the carrageenan/soy protein-treated group compared to both 24 and 48 hours, it remained significantly lower than the viability of untreated cells, with a viability percentage of 86.86%. These changes were statistically significant (p-value=0.0175) (Figure 3d). Furthermore, the carrageenan and soy protein mixture induced a significant increase in overall apoptosis of HCT-116 cells after 72 hours compared to the baseline of the untreated control group and the 48-hour treatment (p-value=0.0000). Interestingly, the level of apoptosis in the CS-HCT group cells showed no significant change after either 24 or 72 hours of treatment (Figure 3e). Meanwhile, the mixture of carrageenan and soy protein treatment showed a non-significant change in early apoptosis levels compared to its levels observed after 0 hours, 24 hours, and 48 hours of treatment (Figure 3f). On the other hand, the late apoptosis level after 48 hours of exposure to the mixture of carrageenan and soy protein still showed a significant decline compared to the level after 24 hours of treatment (p-value = 0.0008). However, no significant changes were observed compared to the 0-hour and 48-hour time points, as indicated by the data presented in Figures 3f and 3g.
Natural products such as carrageenan and soy protein have garnered considerable interest in cancer therapeutics due to their targeted effects on cancer cells and minimal adverse effects [27]. This growing interest reflects the exploration of various natural products for their potential anti-cancer properties. Remarkably, approximately 60% of approved cancer drugs have their origins in natural sources, underscoring their significant role in medicine [5, 17]. The results of the study demonstrate a time-dependent effect of the carrageenan and soy protein treatment on the viability of HCT-116 cells. The findings indicate that the mixture of carrageenan and soy protein has the potential to significantly reduce the viability of these cells. Throughout the 48 and 72-hour treatment periods, the viability of the treated group remained significantly lower compared to the untreated control group.
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
|
|
e) |
|
|
|
f) |
|
|
|
g) |
|
Figure 1. Presents the characterization of the HCT cell line following a 24-hour treatment using a mixture of soy protein and carrageenan. |
The two groups being compared in this study are the untreated control group (UHCT) and the treated group with carrageenan and soy protein (CS-HCT). To determine the significance between these groups, statistical analysis was performed using one-way ANOVA with a significance level of P < 0.05. The expression profile of the CXCR-4 gene in both the treated and untreated HCT cell groups is illustrated in a graph (a). Graph (b) specifically shows the expression profile of the CXCR-4 gene in the CS-HCT treated group after 0 hours. Morphological changes in HCT cells after treatment are depicted in a photograph (c), where black arrows indicate normal features of HCT cells, such as a round nucleus, and red arrows indicate observed cell shrinkage and membrane blebbing in treated HCT cells. Graph (d) displays alterations in cell viability of HCT cells treated with carrageenan and soy protein. The overall apoptosis levels of HCT cells after treatment with carrageenan and soy protein are presented in two graphs (e and f), one showing overall apoptosis levels and the other showing early apoptosis levels. Another graph (g) demonstrates the overall apoptosis levels of HCT cells concerning the late apoptosis level after treatment with carrageenan and soy protein.
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
|
|
e) |
|
|
|
f) |
|
|
|
g) |
|
Figure 2. Characterization of HCT cell line following 48-hour treatment with a mixture of soy protein and carrageenan. |
The experiment had two groups - the untreated control group (UHCT) and the treated group with carrageenan and soy protein (CS-HCT). A statistical analysis was conducted using one-way ANOVA with a significance level of P < 0.05 to determine the significance between the two groups. Graph A displays the expression profile of the CXCR-4 gene in both the treated and untreated HCT cell groups. Graph B shows the expression profile of the CXCR-4 gene specifically in the CS-HCT treated group after 0 hours and 24 hours. Morphological changes in HCT cells after treatment are depicted in graph C, where black arrows indicate normal features of HCT cells and red arrows indicate observed condensation and fragmentation of the nucleus in treated HCT cells. Graph D shows alterations in the cell viability of HCT cells treated with carrageenan and soy protein, while graph E presents the overall apoptosis levels of HCT cells after treatment. Graph F shows the overall apoptosis levels of HCT cells concerning the early apoptosis level following treatment with carrageenan and soy protein, and graph G demonstrates the overall apoptosis levels of HCT cells concerning the late apoptosis level after treatment with carrageenan and soy protein.
|
|
|
a) |
|
|
|
b) |
|
|
|
c) |
|
|
|
d) |
|
|
|
e) |
|
|
|
f) |
|
|
|
g) |
|
Figure 3. Characterization of HCT cell line following 72-hour treatment with a mixture of soy protein and carrageenan. |
The untreated control group is referred to as UHCT, while the group treated with carrageenan and soy protein is called CS-HCT. To assess the significance between the treated and untreated cells, statistical analysis was conducted using one-way ANOVA at a significance level of P < 0.05. The graph (a) depicts the expression profile of the CXCR-4 gene in both treated and untreated HCT cell groups. Graph (b) illustrates the expression profile of the CXCR-4 gene specifically in the CS-HCT treated group after 0 hours and 24 hours. Meanwhile, the morphological changes in HCT cells after treatment are shown in graph (c), with black arrows indicating normal features of HCT cells, such as a round nucleus, and red arrows showing observed condensation and fragmentation of the nucleus in treated HCT cells. The graph (d) displays changes in cell viability of HCT cells treated with carrageenan and soy protein. Furthermore, the graph (e) presents the overall apoptosis levels of HCT cells following treatment with carrageenan and soy protein. Additionally, graph (f) shows the overall apoptosis levels of HCT cells concerning the early apoptosis level after treatment with carrageenan and soy protein. Lastly, graph (g) demonstrates the overall apoptosis levels of HCT cells concerning the late apoptosis level following treatment with carrageenan and soy protein.
This suggests that carrageenan and soy protein could be effective agents for decreasing cell viability in HCT-116 cells. Moreover, the observed morphological changes in the cells, which began at 24 hours and intensified until 72 hours of treatment, support the induction of apoptotic cell death by the carrageenan and soy protein mixture. These changes, along with the significant increase in overall apoptosis compared to the control group, provide further evidence of the treatment's ability to induce apoptosis in the treated cells. The effect of carrageenan and soy protein on HCT-116 cells may be explained by several potential mechanisms [28]. One possible mechanism is the induction of programmed cell death, or apoptosis, in the cells. Additionally, carrageenan and soy protein may hinder cell proliferation by interfering with cell cycle progression or disrupting growth factor signaling pathways [29]. Another mechanism could involve the disruption of cell membrane integrity, either through physical interactions between the food additives and the cell membrane or by activating signaling pathways associated with the membrane. Furthermore, carrageenan and soy protein might induce oxidative stress in the cells, leading to cellular damage and eventual cell death [30].
Cytotoxicity is the process by which damaged or unnecessary cells are naturally eliminated from the body. In cancer cells, apoptosis, the programmed cell death, is often disrupted, leading to uncontrolled growth. Inducing apoptosis in cancer cells is a common strategy for cancer treatment [31]. The current treatment resulted in noticeable changes in cell morphology resembling apoptosis, such as cell shrinkage, membrane blebbing, and cell fragmentation with condensed or fragmented nuclei [32]. These findings suggest that the treatment induces programmed cell death or apoptosis, indicating its potential as a cancer therapy. The cytotoxicity of the carrageenan/soy protein mixture may be attributed to the physical properties of the mixture [33]. Carrageenan might interact with the negatively charged cell membrane of HCT cells, causing damage and triggering apoptosis. Similarly, the soy protein may exert toxic effects on the cells through mechanisms that are not yet fully understood [34]. This study demonstrated a significant increase in total apoptosis in HCT cells treated with carrageenan and soy protein at 24hrs, 48hrs, and 72hrs compared to the initial time point. The effect on total apoptosis was more pronounced at later time points [35], with a significant increase observed at 24hrs and 72hrs compared to 48hrs according to the research.
The study revealed that the combination treatment of carrageenan and soy protein had a time-dependent effect on early apoptosis levels in HCT cells, which exhibited the most significant increase at 48 hours compared to 24 and 72 hours. This suggests that the treatment's impact on apoptosis may vary at different time points, potentially due to the activation of specific signaling pathways associated with programmed cell death [31]. It is important to consider that factors beyond the scope of this study, such as the specific mechanisms underlying apoptosis induction in HCT cells, could influence the observed fluctuations in early apoptosis levels [36]. Nevertheless, the findings emphasize the significance of treatment duration in effectively inducing apoptosis and suggest potential therapeutic applications in cancer treatment [32].
Late apoptosis, an irreversible form of programmed cell death, plays a crucial role in maintaining tissue homeostasis and eliminating damaged or abnormal cells from the body [37]. The current study demonstrated that the combination treatment of carrageenan and soy protein had a notable impact on late apoptosis levels in HCT cells. After 24 hours, there was a significant increase in late apoptosis compared to the control group, but no significant differences were observed between the 48 and 72-hour time points. Additionally, there was no statistical significance at 72 hours compared to the initial 0-hour measurement. These findings suggest that the treatment activated specific signaling pathways that led to the induction of late apoptosis; however, the sustained effect over time was not observed [38]. Possible explanations for the lack of significance at 48 and 72 hours could be the activation of compensatory mechanisms or the clearance of apoptotic cells by phagocytes [39].
The chemokine receptor CXCR-4 plays a role in colon cancer, as it promotes tumor cell proliferation, metastasis, and angiogenesis while also being associated with advanced disease stages and poor prognosis [40]. Although reducing CXCR-4 expression may improve prognosis by inhibiting tumor cell migration and invasion, the mechanisms underlying CXCR-4 downregulation in colon cancer are not fully understood [41]. In the context of cancer, elevated levels of CXCR-4 expression can enhance tumor cell survival, proliferation, angiogenesis, and metastasis. CXCR-4 interacts with its ligand, CXCL12, which is found in various tissues and organs, attracting cancer cells to those sites, and facilitating their colonization. This process is known as chemotaxis [42]. Our research findings demonstrate that the treatment with carrageenan and soy protein has a significant impact on CXCR-4 expression. Notably, there was a significant increase in CXCR-4 transcript levels observed after 24 hours compared to other periods. Specifically, the expression of CXCR-4 was notably higher in the CS-HCT group at 24 hours compared to both the untreated control group and the CS-HCT group treated for 48 and 72 hours. It is worth mentioning that the overall impact of modulating CXCR-4 expression can vary depending on factors such as the specific tumor context, cancer stage, and interactions with other signaling pathways [43]. The significant increase observed in CXCR-4 expression could potentially promote tumor cell proliferation, metastasis, and angiogenesis, which are frequently associated with a more aggressive phenotype and poorer prognosis in colon cancer [41]. On the other hand, if the treatment leads to a decrease in CXCR-4 expression, it may have beneficial effects. Several studies suggest that reducing CXCR-4 expression can inhibit tumor cell migration, invasion, and angiogenesis. Such downregulation may result in decreased tumor aggressiveness and improved prognosis in colon cancer [7, 44]. Multiple potential mechanisms contribute to the downregulation of CXCR-4 in colon cancer, including epigenetic modifications, regulation at the transcriptional level by specific transcription factors, post-translational modifications, and interactions with other signaling pathways implicated in colon cancer [45]. In certain circumstances, CXCR-4 signaling may indirectly impact the expression or activity of proapoptotic genes through cross-talk with other signaling pathways or modulation of cellular survival mechanisms. CXCR-4 signaling has been shown to interact with pathways such as PI3K/Akt and MAPK, which are known to regulate cell survival and apoptosis [7]. Activation of CXCR-4 can lead to the activation of these pathways, promoting cell survival and inhibiting apoptosis. Conversely, blocking CXCR-4 signaling may reduce pro-survival signals and potentially contribute to the induction of apoptosis. In summary, while CXCR-4 signaling itself does not directly stimulate proapoptotic genes, its complex network of interactions with other signaling pathways may indirectly influence apoptotic processes [46]. Also, the current study illustrated that carrageenan/soy protein treatment increased apoptosis levels in HCT cells, which correlated with CXCR-4 mRNA levels. The precise mechanisms and pathways through which CXCR-4 induces apoptosis instead of cell survival [40] are not yet clear. However, CXCR-4 may induce apoptosis by activating the intrinsic apoptotic pathway, which involves the up-regulation of Bak and the down-regulation of antiapoptotic Bcl-XL [46].
CONCLUSION
The study demonstrates the therapeutic potential of manipulating CXCR-4 expression in colon cancer using a combination treatment of carrageenan and soy protein. This treatment leads to a significant reduction in cell proliferation and an increase in apoptosis compared to the control group. The exact mechanisms underlying CXCR-4-induced apoptosis are not fully understood but involve the activation of the intrinsic apoptotic pathway. The combination treatment shows a time-dependent induction of programmed cell death, with the greatest increase in early apoptosis after 48 hours. These findings suggest that the carrageenan and soy protein combination treatment has the potential to effectively induce programmed cell death, particularly in the early stages of apoptosis. This research highlights the promise of using carrageenan and soy protein as complementary therapy in cancer treatment, even in the advanced stages of the disease.
ACKNOWLEDGMENTS: ALL the authors would like to acknowledge King Fahad Center for Medical Research for aiding in the conduction of this study.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: None
References
- Kawaguchi N, Zhang TT, Nakanishi T. Involvement of CXCR4 in normal and abnormal development. Cells. 2019;8(2):185. doi:10.3390/cells8020185
- Bianchi ME, Mezzapelle R. The chemokine receptor cxcr4 in cell proliferation and tissue regeneration. Front Immunol, 2020;11:2109.
- Shi Y, Riese DJ 2nd, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front Pharmacol. 2020;11:574667. doi:10.3389/fphar.2020.574667
- Khare T, Bissonnette M, Khare S. CXCL12-CXCR4/CXCR7 axis in colorectal cancer: Therapeutic target in preclinical and clinical studies. Int J Mol Sci. 2021;22(14):7371. doi:10.3390/ijms22147371
- Wali AF, Majid S, Rasool S, Shehada SB, Abdulkareem SK, Firdous A, et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm J. 2019;27(6):767-77. doi:10.1016/j.jsps.2019.04.013
- Xu SY, Huang X, Cheong KL. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar Drugs. 2017;15(12):388. doi:10.3390/md15120388
- Guo R, Chen M, DingY, Yang P, Wang M, Zhang H, et al. Polysaccharides as potential anti-tumor biomacromolecules -A review. Front Nutr. 2022;9:838179.
- Liu Z, Gao T, Yang Y, Meng F, Zhan F, Jiang Q, et al. Anti-cancer activity of porphyrin and carrageenan from red seaweeds. Molecules. 2019;24(23):4286. doi:10.3390/molecules24234286
- Chen D, Wu XZ, Wen ZY. Sulfated polysaccharides and immune response: Promoter or inhibitor? Panminerva Med. 2008;50(2):177-83.
- do Prado FG, Pagnoncelli MGB, de Melo Pereira GV, Karp SG, Soccol CR. Fermented soy products and their potential health benefits: A review. Microorganisms. 2022;10(8):1606. doi:10.3390/microorganisms10081606
- Kim IS, Yang WS, Kim CH. Beneficial effects of soybean-derived bioactive peptides. Int J Mol Sci. 2021;22(16):8570. doi:10.3390/ijms22168570
- Belobrajdic DP, James-Martin G, Jones D, Tran CD. Soy and gastrointestinal health: A review. Nutrients. 2023;15(8):1959. doi:10.3390/nu15081959
- Badger TM, Ronis MJ, Simmen RC, Simmen FA. Soy protein isolate and protection against cancer. J Am Coll Nutr. 2005;24(2):146S-9. doi:10.1080/07315724.2005.10719456
- Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: A review of in vitro, preclinical, and clinical studies. Nutr Rev. 2009;67(7):398-415. doi:10.1111/j.1753-4887.2009.00213.x
- Hsiao YC, Peng SF, Lai KC, Liao CL, Huang YP, Lin CC, et al. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ Toxicol. 2019;34(4):443-56. doi:10.1002/tox.22698
- Hou S. Genistein: Therapeutic and preventive effects, mechanisms, and clinical application in digestive tract tumor. Evid Based Complement Alternat Med. 2022;2022:5957378. doi:10.1155/2022/5957378
- Islam MR, Akash S, Rahman MM, Nowrin FT, Akter T, Shohag S, et al. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem Biol Interact. 2022;368:110170. doi:10.1016/j.cbi.2022.110170
- Malki A, ElRuz RA, Gupta I, Allouch A, Vranic S, Al Moustafa AE. Molecular mechanisms of colon cancer progression and metastasis: Recent insights and advancements. Int J Mol Sci. 2020;22(1):130. doi:10.3390/ijms22010130
- Pothuraju R, Chaudhary S, Rachagani S, Kaur S, Roy HK, Bouvet M, et al. Mucins, gut microbiota, and postbiotics role in colorectal cancer. Gut Microbes. 2021;13(1):1974795. doi:10.1080/19490976.2021.1974795
- Lee YJ, Cho JM, Sai S, Oh JY, Park JA, Oh SJ, et al. 5-Fluorouracil as a tumor-treating field-sensitizer in colon cancer therapy. Cancers (Basel). 2019;11(12):1999. doi:10.3390/cancers11121999
- Ortiz R, Cabeza L, Arias JL, Melguizo C, Álvarez PJ, Vélez C, et al. Poly(butyl cyanoacrylate) and Poly(ε-caprolactone) Nanoparticles loaded with 5-fluorouracil increase the cytotoxic effect of the drug in experimental colon cancer. AAPS J. 2015;17(4):918-29. doi:10.1208/s12248-015-9761-5
- Janardhanam LSL, Indukuri VV, Verma P, Dusane AC, Venuganti VVK. Functionalized layer-by-layer assembled film with directional 5-fluorouracil release to target colon cancer. Mater Sci Eng C Mater Biol Appl. 2020;115:111118. doi:10.1016/j.msec.2020.111118
- Varghese V, Magnani L, Harada-Shoji N, Mauri F, Szydlo RM, Yao S, et al. FOXM1 modulates 5-FU resistance in colorectal cancer through regulating TYMS expression. Sci Rep. 2019;9(1):1505. doi:10.1038/s41598-018-38017-0
- Hadad SE, Alsolami M, Aldahlawi A, Alrahimi J, Basingab F, Hassoubah S, et al. In vivo evidence: Repression of mucosal immune responses in mice with colon cancer following sustained administration of streptococcus thermophiles. Saudi J Biol Sci. 2021;28(8):4751-61. doi:10.1016/j.sjbs.2021.04.090
- El Hadad S, Zakareya A, Al-Hejin A, Aldahlawi A, Alharbi M. Sustaining exposure to high concentrations of bifidobacteria inhibits gene expression of mouse's mucosal immunity. Heliyon. 2019;5(12):e02866. doi:10.1016/j.heliyon.2019.e02866
- Végran F, Boidot R, Bonnetain F, Cadouot M, Chevrier S, Lizard-Nacol S. Apoptosis gene signature of Survivin and its splice variant expression in breast carcinoma. Endocr Relat Cancer. 2011;18(6):783-92. doi:10.1530/ERC-11-0105
- Maiuolo J, Gliozzi M, Carresi C, Musolino V, Oppedisano F, Scarano F, et al. Nutraceuticals and Cancer: Potential for natural polyphenols. Nutrients. 2021;13(11):3834. doi:10.3390/nu13113834
- Shin DY, Lee WS, Lu JN, Kang MH, Ryu CH, Kim GY, et al. Induction of apoptosis in human colon cancer HCT-116 cells by anthocyanins through suppression of Akt and activation of p38-MAPK. Int J Oncol. 2009;35(6):1499-504. doi:10.3892/ijo_00000469
- Almajali B, Al-Jamal HAN, Taib WRW, Ismail I, Johan MF, Doolaanea AA, et al. Thymoquinone, as a novel therapeutic candidate of cancers. Pharmaceuticals (Basel). 2021;14(4):369. doi:10.3390/ph14040369
- Burton GJ, Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):287-99. doi:10.1016/j.bpobgyn.2010.10.016
- Pfeffer CM, Singh ATK. Apoptosis: A target for anticancer therapy. Int J Mol Sci. 2018;19(2):448. doi:10.3390/ijms19020448
- Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395-417. doi:10.1038/s41571-020-0341-y
- Hefnawy HT, Ramadan MF. Physicochemical characteristics of soy protein isolate and fenugreek gum dispersed systems. J Food Sci Technol. 2011;48(3):371-7. doi:10.1007/s13197-010-0203-1
- Prasedya ES, Miyake M, Kobayashi D, Hazama A. Carrageenan delays cell cycle progression in human cancer cells in vitro demonstrated by FUCCI imaging. BMC Complement Altern Med. 2016;16:270. doi:10.1186/s12906-016-1199-5
- Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007;35(4):495-516. doi:10.1080/01926230701320337
- Ismail NI, Othman I, Abas F, H Lajis N, Naidu R. Mechanism of apoptosis induced by curcumin in colorectal cancer. Int J Mol Sci. 2019;20(10):2454. doi:10.3390/ijms20102454
- Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: Recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486-541. doi:10.1038/s41418-017-0012-4
- Yue J, López JM. Understanding MAPK signaling pathways in apoptosis. Int J Mol Sci. 2020;21(7):2346. doi:10.3390/ijms21072346
- Thorp EB. Mechanisms of failed apoptotic cell clearance by phagocyte subsets in cardiovascular disease. Apoptosis. 2010;15(9):1124-36. doi:10.1007/s10495-010-0516-6
- Mukherjee D, Zhao J. The role of chemokine receptor CXCR4 in breast cancer metastasis. Am J Cancer Res. 2013;3(1):46-57.
- Chatterjee S, Behnam Azad B, Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31-82. doi:10.1016/B978-0-12-411638-2.00002-1
- Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12 / CXCR4 / CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29(4):709-22. doi:10.1007/s10555-010-9256-x
- Shen C, Li J, Li R, Ma Z, Tao Y, Zhang Q, et al. Effects of tumor-derived DNA on CXCL12-CXCR4 and CCL21-CCR7 axes of hepatocellular carcinoma cells and the regulation of sinomenine hydrochloride. Front Oncol. 2022;12:901705. doi:10.3389/fonc.2022.901705
- Zhou W, Guo S, Liu M, Burow ME, Wang G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem. 2019;26(17):3026-41. doi:10.2174/0929867324666170830111531
- Geng Z, Chen M, Yu Q, Guo S, Chen T, Liu D. Histone modification of colorectal cancer by natural products. Pharmaceuticals (Basel). 2023;16(8):1095. doi:10.3390/ph16081095
- Kremer KN, Peterson KL, Schneider PA, Meng XW, Dai H, Hess AD, et al. CXCR4 chemokine receptor signaling induces apoptosis in acute myeloid leukemia cells via regulation of the Bcl-2 family members Bcl-XL, Noxa, and Bak. J Biol Chem. 2013;288(32):22899-914. doi:10.1074/jbc.M113.449926