Archive \ Volume.15 2024 Issue 2
Role of Artificial Intelligence in Pharmacy Practice: A Systematic Review
Abstract
This systematic review explores the evolving role of artificial intelligence (AI) in addressing challenges faced by the pharmaceutical industry, focusing on supply chain disruptions, clinical trials, drug discovery, and clinical trial operations. A comprehensive literature analysis encompassed studies and developments in AI applications within the pharmaceutical sector. The review synthesizes information from various sources, including research articles, reports, and case studies. The pharmaceutical industry has encountered multifaceted challenges, including supply chain disruptions, clinical trial interruptions, and difficulties in drug development. AI emerges as a transformative solution, particularly in supply chain management, clinical trial optimization, drug discovery, and clinical trial operations. Integrating AI models, such as supervised and unsupervised learning, plays a pivotal role in predictive analytics, drug target identification, and optimization of pharmaceutical processes.
The systematic review underscores the transformative impact of AI on pharmacy practice, offering innovative solutions to address challenges in the pharmaceutical industry. The findings suggest that AI applications have the potential to revolutionize supply chain management, streamline clinical trial operations, and expedite drug discovery processes. Continued research and development in AI technologies are essential for optimizing these applications and ensuring their widespread adoption in pharmacy practice.
How to cite:
Download Citation
INTRODUCTION
Many sectors use clinical trial data analysis approaches to improve their success in meeting client requests and expectations. There is no more critical sector when saving lives than the pharmaceutical business. Reacting to medical crises like the current pandemic and other worldwide healthcare issues relies on constant innovation and the use of new technology [1]. Extensive research and development across several areas, including production technology, packaging concerns, and customer-oriented marketing techniques, is usually the foundation of innovation in the pharmaceutical sector [2]. New pharmaceutical developments span the gamut from small molecule drugs to biologics, emphasizing improved stability and high potency to address unfulfilled demands for disease treatment. An area of great worry that will need future study and inquiry is the evaluation of the substantial toxicity levels linked to new medications. Finding the most beneficial and appropriate pharmacological molecules for use in healthcare is one of the main goals. Nevertheless, to meet the medical and healthcare needs of people throughout the globe, the pharmacy business must continue to innovate by using technology-driven approaches [3-5].
Training healthcare workers to enhance their participation in everyday responsibilities is an ongoing need due to the industry's continued demand for competent workers. The pharmaceutical sector places a premium on identifying workplace skill shortages. Recognizing that providing sufficient training may be a substantial problem, it is critical to address the identified gaps successfully via suitable corrective actions. Some authorities have reported that June 2022 was the month in which 41% of supply chain disruptions occurred. The research goes on to say that the second most challenging obstacle to overcome is disruptions in the supply chain. Several pharmaceutical sectors are looking forward to more supply chain innovations and new models to tackle these problems, which may make their businesses more resilient. Clinical trials are among the several activities severely impacted by the 2019 coronavirus illness (COVID-19) pandemic [6].
Supply chain disruptions are more common in the aftermath of pandemics, natural disasters, price fluctuations, cyberattacks, logistical problems, and faulty products. The epidemic's transportation effects have devastated the world's supply chain and sectors. Disputes about whether to use the current or new pricing for commodities or resources cause decision-induced delays in receiving price updates from suppliers, which in turn cause price fluctuations. Problems like rising crime rates and unpredictability in the supply of vital operating and manufacturing resources are new developments stemming from nations' tactics of cross-border economic cooperation. To meet the demands of patients and ensure their compliance, it is necessary to manufacture footprint changes. According to the pharmaceutical industry, the difficulty with maintaining the cold supply chain rendered many COVID-19 vaccinations useless during the pandemic. Inadequate innovation and inaccurate forecasting in business and industrial operations are the main reasons for the interruption in the supply chain due to the delayed reaction. Customer happiness, business image, and potential revenues are all severely affected when there are interruptions in the pharmaceutical industry's supply chain [7].
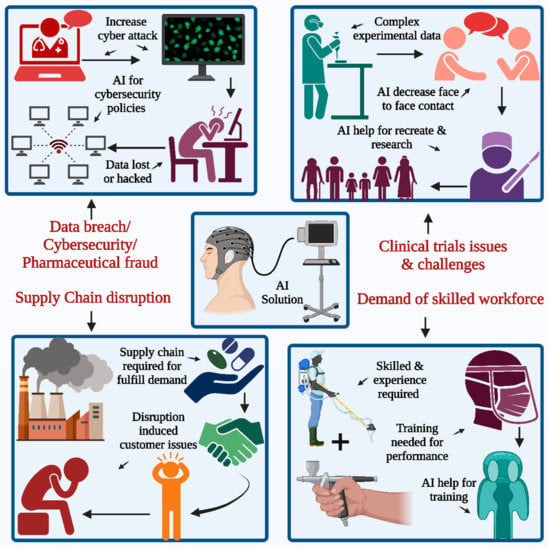
Figure 1 shows that the pharmaceutical industry's supply chain activities will undergo a radical upheaval due to the introduction of AI. Furthermore, it synthesizes a great deal of artificial intelligence research for a few decades to address many supply chain problems. Furthermore, the paper proposes future research directions to improve supply chain management decision-making tools [8].
|
|
|
Figure 1. Potential AI solution to the problems faced by the pharmaceutical business |
Figure 1 shows one potential AI solution to the problems faced by the pharmaceutical business: hiring competent workers is essential for any company that wants to use its knowledge, skills, and ability to innovate new products. The second concerns problems with conducting clinical trials and interruptions in the supply chain. Data breaches and security are becoming significant issues for the sector as cyberattacks increase. Although the pandemic's main effects are fading, they continue to affect therapeutic studies. Artificial intelligence (AI) and virtual platforms are among the latest technologies that many pharmaceutical businesses hope to use. Resuming or recreating these clinical trials using these new technologies might be beneficial, as shown in Figure 1, as they need less contact than face-to-face kinds. The biggest obstacle is the high maintenance expense and the shortage of highly trained personnel. Fourthly, cybersecurity risks and data breaches are significant obstacles to finding a solution via technology [9]. Many pharmaceutical businesses are increasingly worried about the security of their patient's private medical records and other data, which are particularly susceptible to cyberattacks, and the frequency of these assaults has grown in the 21st century. Data fragmentation and disconnected system involvement are two of the biggest problems with conventional clinical trials. The former is usually the consequence of data generated dispersed during the trials, necessitating manual data transcription work for both documents and systems. The trial models aren't innovative enough, so we have to keep fixing repeatedly. Critical aspects in healthcare that need particular attention due to clinical trials include patient recruiting, enrollment, monitoring, retention, and medical adherence. The time-consuming trip to the trial locations impacts patient enrollment, and patients may need to re-enroll in the same study if they visit the sites often [10].
MATERIALS AND METHODS
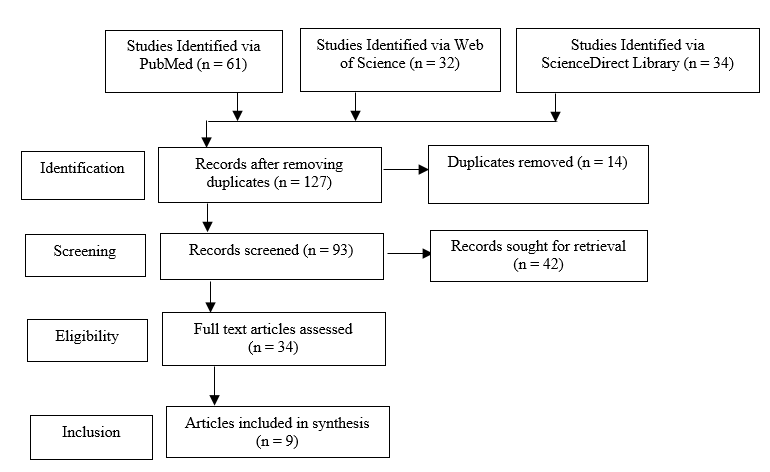
A systematic literature review from 2001 to 2023 was performed using PubMed, Medlin e, and ScienceDirect databases. The keywords were "artificial intelligence, pharmacy practice, pharmaceutical industry". A PRISMA flowchart was used to describe the selection process of the searched-for articles (Figure 2).
Inclusion Criteria
- Randomized control studies, expert opinions, or narrative reviews.
- Published between 2001 and 2023.
- English language of publication.
- In vivo (humans).
Exclusion Criteria
- Systematic reviews or meta-analyses.
- Out of the specified time range.
- Language other than English.
- In vitro.
|
|
|
Figure 2. PRISMA Flow Diagram |
RESULTS AND DISCUSSION
This systematic review explores the pivotal role of artificial intelligence (AI) in addressing challenges within the pharmaceutical industry. The integration of AI is depicted as a transformative force in supply chain management, workforce optimization, and clinical trial disruptions, particularly in the aftermath of the COVID-19 pandemic. The review emphasizes the potential of AI to streamline drug development processes, from target identification to drug discovery, design, and optimization. Furthermore, the application of supervised and unsupervised learning models in various pharmaceutical contexts, such as drug discovery, predictive maintenance, and clinical trial outcomes prediction, is highlighted. The evolving landscape of AI in the pharmaceutical sector promises to revolutionize research and development, offering solutions to longstanding issues and presenting new opportunities for innovation [11].
By incorporating AI into the research design, we can optimize and augment the labor that goes into creating a patient-centric design. Artificial intelligence eliminates the need for human data collectors by automating the collection of the massive volumes of data produced by these clinical studies. These technologies use wearable gadgets and sensors embedded in the patient's body to remotely monitor their vital signs and other important data, allowing healthcare providers to fulfill patients' need for regular in-person contact. During the research, wearable gear powered by AI algorithms delivers real-time information [12].
Effective cybersecurity for both in-office and remote employees requires a new technological platform and solution. Data security and breach strategies also need special consideration. There have been several reports of political fraud, particularly during the pandemic in the last few years, and addressing these situations requires technology. Healthcare fraud may have serious consequences, therefore, it's essential to avoid it. Additionally, it's helpful to stimulate internal talks regarding fraudulent activities regularly.
The Role of AI in Addressing Current Pharmaceutical Challenges
Research on nano molecules is underway in the pharmaceutical sector to improve goods and ensure consumer satisfaction. These molecules have various benefits. Preparing synthetic derivatives is cheap and requires little effort in the chemical synthesis process. Therefore, a wide variety of stable and effective formulations including tiny molecules, are available in the pharmaceutical industry. Innovative small molecules confront competition from generic molecules, complicated data is necessary for their introduction, and clinical studies are also necessary, except for uncommon illness therapy. As a result of these procedures, businesses are under increasing financial pressure to innovate. Amidst the dilemma caused by molecules being too tiny and advances not being widely disseminated, the biomolecular drug business continues to expand quickly. The shape and reactivity of small molecules constitute the foundation of their effects. The building blocks of proteins, known as amino acids, and the building blocks of nucleic acids, known as nucleotides or ribonucleotides, make up biomolecules. The spatial conformation and supramolecular sequence also impact their stability and function. Products from biomolecules, like insulin and adalimumab, have succeeded wildly. Because infusion is the most practical and preferred method of administering these biomolecules, their pharmacokinetics are intricate. Research based on nucleic acids places a premium on pharmacokinetic regulation and molecular stability [13].
Applying AI to Advancements in Drug Delivery and Discovery
Essential objectives include improving the pharmacokinetic exposure of these molecular forms. New technology advancements might be helpful in tackli these obstacles and similar ones. While artificial intelligence (AI) has great promise for the future of medication administration and discovery, it is not without significant limits that human intervention or intellectuals will be needed to decipher the complicated outcomes. Artificial intelligence (AI) relies heavily on datasets for its predictions. However, human intervention is necessary to interpret the findings correctly due to the gray area. When it comes to making predictions and evaluating hypotheses, AI might run into problems with algorithm bias. In addition, the identification of inactive molecules is a typical outcome of docking simulations. Human intervention is still necessary for a rigorous review of these parameters to make successful decisions and conduct cross-verifications to eliminate system bias. Still, AI has a lot of room to grow in practical applications; with enough effort, we should be able to address its shortcomings and make it more trustworthy and effective [14].
Applications of Artificial Intelligence
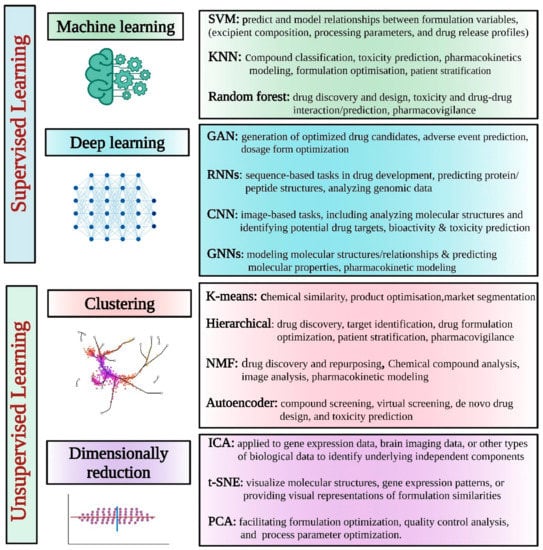
When it comes to artificial intelligence, the approach uses machine learning or portions of it, including deep learning and NLP. Supervised and unsupervised learning are possible, with the algorithm used playing an essential role in both cases. Machine learning approaches may be either supervised or unsupervised.
|
|
|
Figure 3. Various artificial intelligence learning models and techniques for pharmaceutical applications, both supervised and unsupervised. |
The former works with known inputs (features) and outputs (labels or targets), while the latter deals with unknown outputs. Predicting labels or targets as an output from a set of input characteristics is the supervised method. Unsupervised categorization, in contrast, seeks to generate feature-homogeneous groupings.
There has been some investigation into the potential of various AI models to improve various steps in creating medicinal products [15]. Figure 3 details the most often investigated AI models in this field.
Learning via Supervision with AI
The term "supervised learning" describes a subfield of machine learning in which the target outcome is known before algorithm training begins on a labeled dataset. The algorithm determines how to convert the input data into the right output by studying the correlations and patterns in the annotated data. Picture identification, NLP, and modeling predictions are just a few typical uses for this method. With task-driven methods, you start with a set of inputs and work your way out to the results you want. This method trains algorithms for data categorization or result prediction using labeled data. Estimating a label and quantity are the two mainstays of supervised learning: classification and regression. The kind of data in a specific issue area determines the possible strategies for addressing supervised learning problems. These methods include ensemble learning, support vector machines, Naïve Bayes, linear regression, support vector regression, random forest, and K-nearest neighbors. The following are some of its uses in the pharmaceutical sector [6, 7]:
-
- Predicting the activity or characteristics of novel drug candidates is a typical application of supervised learning algorithms in drug discovery and design. The model can discover patterns and correlations between chemical properties and desired outcomes by training on a dataset of known substances and their related activities. This helps in drug discovery and design by allowing the prediction of the activity, potency, or toxicity of new compounds [8].
One use of supervised learning in pharmaceutical production is predictive maintenance and quality control. One way to ensure quality and prevent equipment failure is to train the model using data from manufacturing procedures, device sensors, or quality testing findings. This way, it may learn to anticipate process anomalies, product quality deviations, or equipment failures [9].
One use of supervised learning is the recognition of possible drug targets via the analysis of biological data. It is possible to train the model to recognize trends and find new research objectives by providing data that relate genomic, proteomic, or transcriptome traits to treatment response or development of illness [16].
-
- Health Status Assessment: By analyzing medical records, supervised learning models may forecast how a patient will fare in the future or even diagnose an illness. The model may learn to categorize patients into various diseases or to forecast how a disease will advance or react to therapy by training on labeled datasets that include patient attributes, clinical data, and illness outcomes [17].
Using supervised learning algorithms in pharmacovigilance data allows for the detection and classification of medication-related adverse events. The model may learn to recognize and describe evil occurrences by practicing with labeled reports of such incidents, which helps it to see trends while recognizing possible safety flags [17].
Clinical trial outcome prediction using supervised learning is one use of supervised learning in predictive modeling. The model may learn to anticipate patient reaction, efficiency of treatment, or safety consequences by practicing with previous information from clinical trials, which includes patient attributes, treatment interventions, and trial results. With this data, researchers can better identify patients for trials and improve trial design overall [18].
The pharmaceutical sector may use supervised learning in many other ways. At several points in the pharmaceutical R&D and production processes, supervised learning methods, when coupled with suitable feature selection, data preprocessing, and model assessment, provide valuable insights and bolster choices.
Unsupervised AI Learning
The absence of labels in training data is known as "unsupervised learning," a subfield of machine learning. Its function, instead, is to seek associations and trends in the data autonomously. This method might help you find clusters or hidden structures in your dataset if you're doing exploratory data analysis. As a "data-driven methodology," the goal is to derive meaning from raw, unstructured data. Clustering, dimensionality reduction, information visualization, discovering association rules, and anomaly detection are some of the most common unsupervised activities. Standard methods for unsupervised learning include clustering algorithms (e.g., hierarchical clustering, K-means, K-medoids, single linkage, complete linkage, BOTS), association learning algorithms, feature selection, and extraction techniques (e.g., Pearson correlation, principal component analysis) that are based on the characteristics of the data. Following is an explanation of how unsupervised learning methods in artificial intelligence (AI) might be used in the pharmaceutical industry, specifically for tasks like data visualization, pattern identification, and exploratory analysis [18]:
-
- Clustering: Algorithms for clustering collect data points according to their similarities, enabling the discovery of inherent groups. Gene expression profiles, chemical structures, and patient data are just a few examples of information that might benefit from clustering in the pharmaceutical industry. Finding new classes of chemicals or disorders, better-stratifying patients, and discovering targets may all benefit from this.
Principal component analysis (PCA) and t-distributed stochastic neighbor embedding (t-SNE) are dimensionality reduction approaches that simplify high-dimensional datasets without losing precious information. Visualizing and exploring complicated information, identifying significant variables or characteristics, and supporting decision-making processes are all possible using these tools. Gene expression data, medication activity profiles, and imaging data are just a few examples of pharmaceutical data types that might benefit from dimensionality reduction [19].
One such technique is anomaly detection, which uses algorithms to spot out-of-the-ordinary data items that don't fit the norm. Abnormal event detection, possible safety concern identification, and data quality problem discovery are all areas where anomaly detection has shown promise in the pharmaceutical business. To identify data points or patterns that are out of the ordinary and need more examination, one may use an unsupervised anomaly detection method like isolation forest or the local outlier factor (LOF) [19].
Finding meaningful connections or correlations between elements in a collection is the goal of association rule mining algorithms like the Apriori algorithm. Association rule mining has many potential uses in the pharmaceutical industry, including analyzing medicine-drug interactions, adverse event data, and medication and medical condition co-occurrence patterns. Potential interactions between drugs, medication habits, and pharmacovigilance activities may all be better understood using these methods [20].
Latent Dirichlet allocation (LDA) and other topic modeling algorithms can sift through mountains of text for hidden themes and subjects. One use of topic modeling in the pharmaceutical sector is the identification of critical research subjects, developing trends, or patient feelings from sources such as social media data, clinical trial reports, and scientific literature. This may benefit several fields, including literature mining, competitive intelligence, and patient viewpoint comprehension [11, 21].
In the pharmaceutical industry, unsupervised learning methods provide valuable insights and exploratory analyses. To extract useful information and guarantee the dependability of the results, it is sometimes necessary to use domain knowledge and conduct additional validation when interpreting results from unsupervised learning techniques.
Artificial intelligence has changed the face of drug development and research in several ways. The points that follow are examples of essential AI contributions in this field [6]:
Identifying the Target
AI systems may sift through several kinds of data to find new treatment targets, including genomic, proteomic, and clinical information. Artificial intelligence (AI) aids in developing drugs that can regulate biological processes by revealing disease-associated targets and molecular pathways.
Online Preview
Artificial intelligence allows for rapidly screening large chemical libraries to find potential drugs with a high binding affinity for a particular target. Using AI, scientists may save time and energy by prioritizing and selecting compounds for experimental testing based on predicted binding affinities and simulations of chemical interactions.
The Structure-Activity Relationship (SAR)
Artificial intelligence models can represent the relationship between a chemical compound's molecular structure and its biological function. Because of this, scientists can improve their therapeutic ideas by creating compounds with better pharmacokinetic characteristics, selectivity, and potency.
New Drug Design
Artificial intelligence systems may suggest new chemical compounds like drugs using generative models and reinforcement learning. To help create new medication candidates, AI learns from chemical libraries and experimental data, which increases the chemical space.
Enhancement of Potential Medicines
Efficacy, safety, and pharmacokinetics are just a few aspects AI algorithms may examine and enhance regarding medication candidates. By doing so, scientists can improve the efficacy of medicinal compounds while reducing the likelihood of adverse consequences.
Reuse of Existing Drugs
Artificial intelligence methods can sift through mountains of biological data, searching for current medications with therapeutic promise for various illnesses. Artificial intelligence shortens and lowers the cost of drug research by reusing already-approved medications for other purposes.
Anticipation of Toxic Effects
By studying substances' molecular formulas and properties, AI systems may foretell which drugs will be hazardous. Predicting adverse effects or identifying potentially dangerous structural features are two tasks that machine learning algorithms trained on toxicology databases are capable of doing. In clinical studies, this aids in the prioritization of safer substances and the mitigation of any harmful reactions.
Current Status of AI in Pharmacy Practice
Artificial intelligence and machine learning can significantly enhance various areas of research and development, which could lead to a higher chance of successful drug development. These areas include recognition of new targets, anticipate of protein structures, design and optimization of molecular compounds, knowledge of disease processes, creation of projected and predicted biomarkers, as well as analysis of biometric data from wearable devices, imaging, precision medicine, and most recently, designing, ethical conduct, and evaluation of clinical experiments. Due to the rising dependence on digital technologies for data collecting and site tracking, the COVID-19 pandemic may hasten the adoption of artificial intelligence and machine learning in the conduct of clinical trials.
Clinical Trial Data Analysis
Utilizing natural language processing (NLP) to glean scientific insights from biomedical literature, unstructured EMR, and insurance applications can lead to the discovery of new targets in the pre-clinical realm. Predictive modeling, meanwhile, can foretell protein structures and aid in the design as well as the optimization of molecular compounds, allowing for the selection of more promising drug candidates. Machine learning techniques have come a long way in addressing the "Large p, Small n" issue, which arises when there are more variables ("p") than samples ("n"), thanks to the deluge of high-dimensional data generated by genomics, imaging, and computerized devices that are wearable. Using "big data" from real-world sources, these methods also help with post-marketing research in three ways: (i) improving our knowledge of a drug's benefit-risk profile, (ii) illuminating patterns in treatment sequences, and (iii) pinpointing subsets of patients that might reap more benefits from a particular treatment than others (precision medicine) [6, 8].
The application of artificial intelligence and machine learning in clinical trial operations and data analysis has lagged behind its more sophisticated usage in drug development, translational research, and the pre-clinical phase throughout the last 20 years. Site selection, patient recruiting, trial monitoring, and data collecting are all part of what we call "clinical trial procedures" when we talk about the activities that go into running the clinical trials. The statistical analysis, processing, and management of data pertaining to clinical trial participants is known as clinical trial data analysis. When it comes to the operational side of trials, recruiting patients has proven to be especially difficult; over 30% of phase 3 studies ended early because of enrollment issues and an estimated 80% of trials failed to reach enrollment deadlines [22, 23]. Regulators require important and costly quality control measures, and one of them is trial site tracking, which requires actual travel to locations. Clinical trial tracking is also becoming more expensive, time-consuming, and labor-intensive due to multi-center worldwide studies. Also, there's an excellent chance for AI/ML to positively disrupt the process that has remained primarily unaltered over the previous 20 years: the time it takes to get from the "last subject last visit" trial milestone for the last phase 3 trial to submitting the data package for regulatory clearance. Decreasing this delay would significantly enhance our capacity to expedite the delivery of pharmaceuticals to patients while simultaneously lowering their costs. Intervening steps include finalizing the trial database and locking it, producing the results of the last phase 3 trial analysis (which can include hundreds of summary tables, data listings, and figures), penning the clinical study report, finishing the integrated summary of effectiveness and safety, and lastly, making the data submission package. An increase in the use of digital technologies to gather patient data and a drive toward entirely or partly virtual (or "decentralized") trials are two ways in which COVID-19 might hasten the integration of AI and ML into clinical trial operations. With the use of AI and ML, we can improve patient registration and admission, automate and "smartly" monitor clinical data quality and trial site performance in real-time, and much more. Clinical trial data analysis, research reports development, and regulatory submission data packages are three areas where we anticipate AI/ML to have a revolutionary impact on the field of clinical trial operations and data analytics [24, 25].
Examples of Real-Life Situations
For convenience, we have included an example of past and current research and development usage of AI and ML methodologies below.
Drug Discovery and the Use of DL to Predict Protein Structures and Repurpose Existing Drugs
The three-dimensional (3D) framework of a protein is defined by its one-dimensional (1D) sequence of amino acids, which in turn dictates its molecular mechanism. To cure specific disorders, scientists use what we know about protein structures to deduce how proteins work biologically and to find ways to either activate or inhibit these proteins. Neurodegenerative disorders, including Alzheimer's, Parkinson's, Huntington's, and amyotrophic lateral sclerosis, are among those known to include protein misfolding [5]. Type II diabetes is among these diseases. Developing algorithms that can adequately predict 3D protein structures will significantly benefit drug development and our understanding of protein-folding illnesses since there is now a knowledge gap between the 1D string of amino acid sequences and the 3D structure of a protein. DeepMind (Google) created AlphaFold, an artificial intelligence network that uses the sequence of amino acids to predict the three-dimensional structure of a protein [26, 27]. To deduce the protein's structure from its sequence, a DL method was used. The core part of AlphaFold is a convolutional neural network that was trained using structures from the Database of Proteins. Its purpose is to forecast the distances between each pair of residues in a protein sequence, providing a probability estimate for a 64 by 64 area on the distance map. By tiling these areas together, we can get spacing estimates for the whole protein, which we can use to build a framework for the protein that matches these predictions. The SARS-CoV-2 membrane protein, Nsp2, Nsp4, Nsp6, and Papain-like proteinase (C terminal domain) are five understudied SARS-CoV-2 targets that AlphaFold predicted their structures for in 2020. This could lead to a better knowledge of these biological systems [8].
In order to find powerful FDA-approved drugs that could block the functions of SARS-CoV-2's core proteins, Vora et al. [6] created a deep learning-based drug-target interaction prediction model named Molecule Transformer-Drug Target Interaction (MT-DTI). This model can predict binding affinities based on the chemical and amino acid sequences of a target protein without their structural information. Several well-known antiviral drugs were computationally identified by Aggarwal et al. and Wani et al. as having inhibitory potency against the SARS-CoV-2 3C-like proteinase. These drugs, including atazanavir, remdesivir, efavirenz, ritonavir, and dolutegravir, could be repurposed and tested in clinical trials as potential treatments for SARS-CoV-2 infection [28, 29].
CONCLUSION
In conclusion, this systematic review explores the pivotal role of artificial intelligence (AI) in addressing challenges within the pharmaceutical industry. The integration of AI is depicted as a transformative force in supply chain management, workforce optimization, and clinical trial disruptions, particularly in the aftermath of the COVID-19 pandemic. The review emphasizes the potential of AI to streamline drug development processes, from target identification to drug discovery, design, and optimization. Furthermore, the application of supervised and unsupervised learning models in various pharmaceutical contexts, such as drug discovery, predictive maintenance, and clinical trial outcomes prediction, is highlighted. The evolving landscape of AI in the pharmaceutical sector promises to revolutionize research and development, offering solutions to longstanding issues and presenting new opportunities for innovation.
ACKNOWLEDGMENTS: None
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: None
References
- Ranchon F, Chanoine S, Lambert-Lacroix S, Bosson JL, Moreau-Gaudry A, Bedouch P. Development of artificial intelligence powered apps and tools for clinical pharmacy services: A systematic review. Int J Med Inform. 2023;172:104983.
- Del Rio-Bermudez C, Medrano IH, Yebes L, Poveda JL. Towards a symbiotic relationship between big data, artificial intelligence, and hospital pharmacy. J Pharm Policy Pract. 2020;13(1):75.
- Alowais SA, Alghamdi SS, Alsuhebany N, Alqahtani T, Alshaya AI, Almohareb SN, et al. Revolutionizing healthcare: The role of artificial intelligence in clinical practice. BMC Med Educ. 2023;23(1):689.
- Kulkov I. The role of artificial intelligence in business transformation: A case of pharmaceutical companies. Technol Soc. 2021;66:101629.
- Roosan D, Wu Y, Tatla V, Li Y, Kugler A, Chok J, et al. Framework to enable pharmacist access to health care data using Blockchain technology and artificial intelligence. J Am Pharm Assoc. 2022;62(4):1124-32.
- Vora LK, Gholap AD, Jetha K, Thakur RRS, Solanki HK, Chavda VP. Artificial intelligence in pharmaceutical technology and drug delivery design. Pharmaceutics. 2023;15(7):1916.
- Khatib MM, Ahmed G. Robotic pharmacies potential and limitations of artificial intelligence: A case study. Int J Bus Innov Res. 2020;23(3):298-312.
- Struble TJ, Alvarez JC, Brown SP, Chytil M, Cisar J, DesJarlais RL, et al. Current and future roles of artificial intelligence in medicinal chemistry synthesis. J Med Chem. 2020;63(16):8667-82.
- Baines D, Nørgaard LS, Babar ZU, Rossing C. The fourth industrial revolution: Will it change pharmacy practice? Res Social Adm Pharm. 2020;16(9):1279-81.
- Trenfield SJ, Awad A, McCoubrey LE, Elbadawi M, Goyanes A, Gaisford S, et al. Advancing pharmacy and healthcare with virtual digital technologies. Adv Drug Deliv Rev. 2022;182:114098.
- Kolluri S, Lin J, Liu R, Zhang Y, Zhang W. Machine learning and artificial intelligence in pharmaceutical research and development: A Review. AAPS J. 2022;24(1):19.
- Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26(1):80-93.
- Choudhury A, Asan O. Role of artificial intelligence in patient safety outcomes: Systematic literature review. JMIR Med Inform. 2020;8(7):e18599.
- Kaplan A, Cao H, FitzGerald JM, Iannotti N, Yang E, Kocks JWH, et al. Artificial intelligence/machine learning in respiratory medicine and potential role in asthma and COPD diagnosis. J Allergy Clin Immunol Pract. 2021;9(6):2255-61.
- Eaneff S, Obermeyer Z, Butte AJ. The case for algorithmic stewardship for artificial intelligence and machine learning technologies. JAMA. 2020;324(14):1397-8.
- Shuaib A, Arian H, Shuaib A. The increasing role of artificial intelligence in health care: Will robots replace doctors in the future? Int J Gen Med. 2020;13:891-6.
- Angehrn Z, Haldna L, Zandvliet AS, Gil Berglund E, Zeeuw J, Amzal B, et al. Artificial intelligence and machine learning applied at the point of care. Front Pharmacol. 2020;11:759.
- Rezaei M, Rahmani E, Khouzani SJ, Rahmannia M, Ghadirzadeh E, Bashghareh P, et al. Role of artificial intelligence in the diagnosis and treatment of diseases. Kindle. 2023;3(1):1-60.
- van der Lee M, Swen JJ. Artificial intelligence in pharmacology research and practice. Clin Transl Sci. 2023;16(1):31-6.
- Selvaraj C, Chandra I, Singh SK. Artificial intelligence and machine learning approaches for drug design: Challenges and opportunities for the pharmaceutical industries. Mol Divers. 2022;26(3):1893-913.
- Huysentruyt K, Kjoersvik O, Dobracki P, Savage E, Mishalov E, Cherry M, et al. Validating intelligent automation systems in pharmacovigilance: Insights from good manufacturing practices. Drug Saf. 2021;44(3):261-72.
- Elbadawi M, McCoubrey LE, Gavins FKH, Ong JJ, Goyanes A, Gaisford S, et al. Harnessing artificial intelligence for the next generation of 3D printed medicines. Adv Drug Deliv Rev. 2021;175:113805.
- Shaheen MY. Applications of artificial intelligence (AI) in healthcare: A review. ScienceOpen Preprints. 2021.
- Liu Z, Wu Z, Hu M, Zhao B, Zhao L, Zhang T, et al. Pharmacygpt: The ai pharmacist. arXiv preprint arXiv:2307.10432. 2023.
- Zhavoronkov A, Vanhaelen Q, Oprea TI. Will artificial intelligence for drug discovery impact clinical pharmacology? Clin Pharmacol Ther. 2020;107(4):780-5.
- Lou B, Wu L. AI on drugs: Can artificial intelligence accelerate drug development? Evidence from a large-scale examination of bio-pharma firms. Evidence from a Large-scale Examination of Bio-pharma Firms, (March 15, 2021). MISQ Forthcoming. 2021.
- Lee D, Yoon SN. Application of artificial intelligence-based technologies in the healthcare industry: Opportunities and challenges. Int J Environ Res Public Health. 2021;18(1):271.
- Aggarwal N, Ahmed M, Basu S, Curtin JJ, Evans BJ, Matheny ME, et al. Advancing artificial intelligence in health settings outside the hospital and clinic. NAM Perspect. 2020;2020.
- Wani SUD, Khan NA, Thakur G, Gautam SP, Ali M, Alam P, et al. Utilization of artificial intelligence in disease prevention: Diagnosis, treatment, and implications for the healthcare workforce. Healthcare (Basel). 2022;10(4):608.