Archive \ Volume.15 2024 Issue 4
Investigation of Pharmacological and Wound-Healing Properties of Zinc Oxide Nanoparticles
Abstract
A comparative analysis of the specific pharmacological activity and wound-healing properties of zinc oxide nanoparticles (ZnO-NPs) was carried out. It has been shown that ZnO-NP forms reduce the severity of formalin paw edema in mice and the exudative reaction in "felt" granuloma in rats. Notably, the effectiveness of the ointment form exceeded the effectiveness of the gel form. The ability of ointment and gel based on ZnO-NPs has been shown to significantly reduce the recovery time of hemorrhagic soft tissue injuries in rats and, unlike medicinal forms of heparin, to increase the blood clotting time of rabbits by 1.25 times and 1.19 times, respectively. Thus, ointment and gel containing ZnO-NPs are potential medicines with anti-inflammatory, anticoagulant properties and the property of shortening the recovery time of hemorrhagic soft tissue injuries. It is concluded that the therapeutic effect of samples containing ZnO-NPs, due to the ability of these nanoparticles to penetrate the skin epithelium, providing regenerating and fibrinolytic activity, blocking the activity of inflammatory mediators and providing, including the systemic effect on blood clotting.
How to cite:
Download Citation
INTRODUCTION
Modern medicine pays increasing attention to the creation of new highly effective and safe medicines [1]. For the most part, the use of drugs presented as products of chemical synthesis, as well as animal or plant origin, is accompanied by side effects, which often limits their clinical use [2-4]. The problem of finding new potential medicines, including those based on natural raw materials, remains urgent. At the same time, it is important to search for drugs that exhibit a multifaceted effect (including anti-inflammatory, wound healing, anti-burn, antihemorrhagic, and anti-clotting) when applied to the skin for the treatment of injuries [5-7].
Currently, zinc oxide nanoparticles (ZnO-NPs) are widely used in various fields due to their special physico-chemical properties: diverse morphology, large surface area to volume ratio, powerful antibacterial activity, excellent biocompatibility, environmental friendliness, cost-effectiveness and low toxicity [8-11]. ZnO-NPs have unique optical, chemical, semiconductor, photocatalytic, electrically conductive, and piezoelectric properties [12-15]. They are widely used to treat various skin conditions, have wound-healing activity, anti-cancer properties, and exhibit antimicrobial activity against various microorganisms [16-21].
Therefore, there is a scientific and practical interest in the application of ZnO-NPs in formulations of modern drugs with multifaceted pharmacological and wound-healing properties. Thus, this study aimed to evaluate the prospects for the development of drugs based on ZnO-NPs in the case of skin application.
MATERIALS AND METHODS
The experiments were carried out on 100 white non-harmless mice weighing 20.2±0.4 g, 240 white non-harmless rats weighing 210±4 g, and 20 rabbits weighing 3000±10 g. The studies were conducted following the rules of high-quality laboratory practice in conducting preclinical studies in the Russian Federation. In vivo experiments were performed in compliance with national and international requirements for the maintenance and humane treatment of animals [22]. The objects of the study were ZnO-NPs produced by the Institute of General Physics (Moscow, Russia), as well as "Fastum” ointment and gel for external use (Biopharm, Moscow, Russia).
In the work, the doses of the studied ointment and gel were determined taking into account the interspecific recalculation of doses from humans (4g/70kg = 57.1 mg/kg) per mouse (1:11), rat (1:7) and rabbit (1:3.2) [23]. The resulting doses amounted to 630 mg/kg for mice, 400 mg/kg for rats, and 183 mg/kg for rabbits. ZnO-NPs were used in the same doses as a 1% solution with 0.9% NaCl. Diclofenac gel (Biopharm, Moscow, Russia) and Heparin ointment (BioProtect, Gomel, Belarus) were used as controls and applied to animals at the same doses.
Anti-inflammatory activity was evaluated on a model of formalin paw edema in mice with subplantar injection of 0.1 mL of 2% aqueous formalin solution into the hind paw [24]. The experimental animals applied the studied samples to the sole of the paw with light rubbing daily for 10 days. The control group was given an isotonic sodium chloride solution. The severity of edema was assessed by measuring the thickness of the foot before and 4 hours after the administration of formalin [25-27]. The evaluation of anti-inflammatory activity was also carried out on a model of "felt" granuloma in rats [28]. Chronic proliferative inflammation was caused by the implantation of sterilized felt pieces weighing 40±2 mg under the skin of the medial part of the back [29, 30]. The studied samples were applied daily to the trimmed area with light rubbing. On the 8th day after the operation, pieces of felt with granulation tissue formed around them were removed, weighed, and dried to a constant mass. The proliferative and exudative reactions were calculated and expressed in % [31].
The therapeutic effect in hemorrhagic lesions was evaluated on non-harmless white rats. In experimental animals, the hair covering on a part of the skin of the back was removed. Hemorrhagic soft tissue injuries using local anesthesia (lidocaine) were caused by applying a standard blunt blow of fixed force [32, 33]. The examined samples were applied to rats daily for 6 days with light rubbing until the hematomas completely disappeared.
The wound healing effect was studied on white nonlinear rats. Previously, the hair covering a part of the skin of the back with an area of 3x3 cm2 was removed from the experimental animals. Layered skin wounds were applied using a 225 mm2 stencil using local anesthesia (lidocaine) [34]. The tested samples were applied daily to the skin of experimental animals until complete healing.
The anticoagulation effect was evaluated on rabbits when applied to experimental animals for 10 days with light rubbing of the test samples onto a skin area with a hairline removed with an area of 4x4 cm2 [35]. Before the experiment and 10 days later, blood was taken from the marginal vein of the ear to record the time of blood clotting according to the hemocoagulation analysis using AGCM 1-01 device (Lumex, St. Petersburg, Russia).
Statistical data processing was performed using the software package Microsoft Office EXCEL 2010 and STATISTICA 12. The presence of a normal distribution of data was checked using the Kolmogorov–Smirnov criterion. The parameters of the normal distributions of features in the samples were described in the format M±m (mean value ± standard error of the mean). The statistical reliability of the differences in research results between the groups was checked by the Student's t-test.
RESULTS AND DISCUSSION
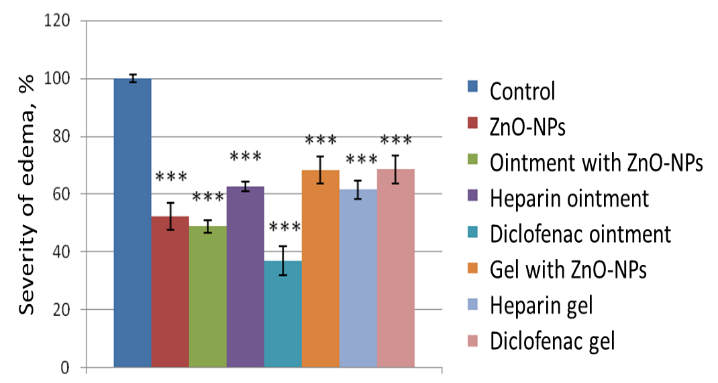
With subplantar administration of 0.1 mL of 2% formalin solution, experimental mice develop pronounced paw edema, as evidenced by a significant increase in its volume. 4 hours after the injection of formalin, the paw volume in the experimental animals of the control group increased by 72.1 ± 2.8%. With the skin application of ZnO-NPs, there was a decrease in the severity of formalin-induced paw edema in mice to 52.28±4.60%, which is 1.38 times (p<0.05) less than in the control. The severity of the anti-inflammatory effect of 1% of the ointment with ZnO-NPs corresponded to the activity of aqueous extraction of ZnO-NPs, whereas the gel with ZnO-NPs acted weaker (Figure 1). This was also manifested in relation to the activities of the ointment and diclofenac gel.
|
|
|
Figure 1. The effect of control and experimental substances on the severity of formalin edema of the paws of mice. Note: *, ** and *** – the differences with the control are significant at p<0.05, 0.01 and 0.001, respectively |
Thus, diclofenac ointment showed the greatest effect on decongestant action (63.22±4.96%), 1.64 times more pronounced (p<0.05) than diclofenac gel (38.5±4.8%). ZnO-NPs and ZnO-NPs-based ointment also showed a fairly high reliable effect, amounting to 47.72±4.6% and 51.3±2.2%, respectively, against 31.74±4.6% for a ZnO-NPs-based gel, 1.6 times more pronounced than for a gel. The smallest but reliable effect was found for heparin ointment and gel, amounting to 37.5±1.7% versus 38.5.5±3.18%.
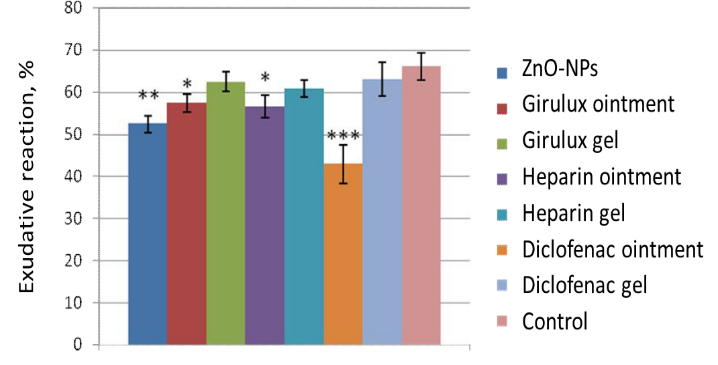
On day 8, the exudative and proliferative reactions in "felt" granuloma in rats amounted to 66.1±3.3% and 9.4±0.8%, respectively. When evaluating the proliferative reaction in "felt" granuloma in rats, there were no significant differences from the control group on day 8. At the same time, for the exudative reaction, a significant decrease was revealed when using ZnO-NPs substance, ZnO-NPs ointment, heparin ointment, and diclofenac ointment with 66.1±3.3% in the control to 52.5±2.0 %, 57.5±4.1 %, 56.6±2.7 % and 42.9±4.6%, respectively. For the gel based on ZnO-NPs, heparin gel, and diclofenac gel, there was only a tendency to decrease this indicator (Figure 2).
Thus, an analysis of the results of an experimental study of anti-inflammatory activity showed that when ZnO-NPs were applied to a model of felt granuloma in rats, there was a decrease in the severity of the exudative reaction without affecting the proliferative one.
|
|
|
Figure 2. The effect of control and experimental substances on the severity of the exudative reaction in "felt" granuloma in rats. |
When evaluating the therapeutic effect of the studied samples for hemorrhagic soft tissue injuries in rats, it was found that the time of resorption of hematomas using ZnO-NPs significantly decreased by an average of 1.22 times, and the gel with ZnO-NPs – by 1.16 times. The data presented in Table 1 indicate that ZnO-NPs in free and gel forms lead to a significant reduction in the recovery time of hemorrhagic soft tissue injuries in rats [36-38].
|
Table 1. The effect of Girulux gel and ZnO-NPs on the course of hemorrhagic soft tissue injuries in rats |
||
|
Sample |
Terms of tissue recovery after hemorrhagic damage, days |
|
|
Results |
Total change, % |
|
|
ZnO-NPs |
3.6±0.2* |
-18.2±4.5* |
|
Gel with ZnO-NPs |
3.8±0.2* |
-13.6±4.5* |
|
Heparin Gel |
3.9±0.3 |
-11.4±6.8 |
|
Control |
4.4±0.2 |
-0.0±4.5 |
Notably, there were no significant differences in the healing rate of skin wounds under the action of ZnO-NPs and ointment with leech extract, but there was a tendency to accelerate the healing process by 0.4 days (4.8%) and 0.3 days (3.6%), respectively.
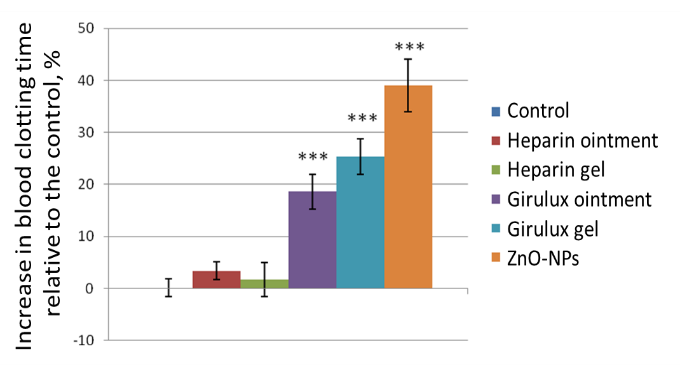
Skin application of ZnO-NPs to rabbits leads to a significant increase in the blood clotting time of experimental animals by an average of 40%, ointment with ZnO-NPs by 25%, and when using gel with ZnO-NPs by 19%. At the same time, heparin preparations proved ineffective in this setting of the experiment (Figure 3).
|
|
|
Figure 3. Effect of substance, ointment, gel with ZnO-NPs, and heparin on blood clotting time in rabbits. |
Thus, ZnO-NPs, as well as Girulux gel and Girulux ointment showed anti-inflammatory, anticoagulant properties and the ability to accelerate the resorption of hemorrhagic soft tissue injuries. The results obtained are in line with recent data reported by other researchers [39-41].
Taking into account the fact that earlier it was shown that the acceleration of healing of burn wounds in rats when applying ZnO-NPs was on average 1.2 times faster than in the control with a significant (3 times) acceleration of the rate of epithelization of burn wounds from 5 days after injury [42-46]. Probably, the therapeutic effect of samples containing ZnO-NPs, due to the ability of these nanoparticles to penetrate the skin epithelium, providing regenerating and fibrinolytic activity, blocking the activity of inflammatory mediators and providing, including the systemic effect on blood clotting [47-50].
CONCLUSION
ZnO-NPs, when applied to mice in a dose of 630 mg/kg, inhibit paw edema with the introduction of formalin. Moreover, diclofenac ointments with ZnO-NPs and heparin exceed the activity of similar gels in terms of the effectiveness of inflammation suppression. In the model of felt granuloma in rats, the ZnO-NPs substance reduces the exudative reaction during course application. ZnO-NPs and ZnO-NPs gel, unlike heparin gel, significantly reduce the tissue recovery time for hemorrhagic lesions in rats. Daily for 10 days, course skin application of ZnO-NPs ointment to rabbits at a dose of 183 mg/kg leads to an increase in the blood clotting time of experimental animals by an average of 1.25 times, and when using gel by 1.2 times, unlike heparin preparations. Ointment and gel with ZnO-NPs when applied on the skin of experimental animals have a complex anti-inflammatory effect, accelerate tissue repair after hemorrhagic damage, reduce the rate of blood clotting, and are potential medicines.
ACKNOWLEDGMENTS: None
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: All studies were performed following the Guidelines for the preclinical study of medicines of the Russian Federation and approved by the Ethics Commission (Protocol 3 dated by Aug 3, 2024).
References
- Hampton JP, Hommer K, Musselman M, Bilhimer M. Rapid sequence intubation and the role of the emergency medicine pharmacist: 2022 update. Am J Health Syst Pharm. 2023;80(4):182-95. doi:10.1093/ajhp/zxac326
- Weersma RK, Zhernakova A, Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69(8):1510-9. doi:10.1136/gutjnl-2019-320204
- Meneghini M, Bestard O, Grinyo JM. Immunosuppressive drugs modes of action. Best Pract Res Clin Gastroenterol. 2021;54:101757. doi:10.1016/j.bpg.2021.101757
- Luethi D, Liechti ME. Designer drugs: Mechanism of action and adverse effects. Arch Toxicol. 2020;94(4):1085-133. doi:10.1007/s00204-020-02693-7. Epub 2020 Apr 6. Erratum in: Arch Toxicol. 2022;96(5):1489. doi:10.1007/s00204-022-03244-y
- Doytchinova I. Drug design-past, present, future. Molecules. 2022;27(5):1496. doi:10.3390/molecules27051496
- Large DE, Abdelmessih RG, Fink EA, Auguste DT. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv Drug Deliv Rev. 2021;176:113851. doi:10.1016/j.addr.2021.113851
- Zagotto G, Bortoli M. Drug design: Where we are and future prospects. Molecules. 2021;26(22):7061. doi:10.3390/molecules26227061
- Umair Hassan M, Huang G, Haider FU, Khan TA, Noor MA, Luo F, et al. Application of zinc oxide nanoparticles to mitigate cadmium toxicity: Mechanisms and future prospects. Plants (Basel). 2024;13(12):1706. doi:10.3390/plants13121706
- Pushpalatha C, Suresh J, Gayathri VS, Sowmya SV, Augustine D, Alamoudi A, et al. Zinc Oxide nanoparticles: A review on its applications in dentistry. Front Bioeng Biotechnol. 2022;10:917990. doi:10.3389/fbioe.2022.917990
- Wiesmann N, Tremel W, Brieger J. Zinc oxide nanoparticles for therapeutic purposes in cancer medicine. J Mater Chem B. 2020;8(23):4973-89. doi:10.1039/d0tb00739k
- do Carmo Neto JR, Guerra RO, Machado JR, Silva ACA, da Silva MV. Antiprotozoal and anthelmintic activity of zinc oxide nanoparticles. Curr Med Chem. 2022;29(12):2127-41. doi:10.2174/0929867328666210709105850
- Mandal AK, Katuwal S, Tettey F, Gupta A, Bhattarai S, Jaisi S, et al. Current research on zinc oxide nanoparticles: Synthesis, characterization, and biomedical applications. Nanomaterials (Basel). 2022;12(17):3066. doi:10.3390/nano12173066
- Jha S, Rani R, Singh S. Biogenic zinc oxide nanoparticles and their biomedical applications: A review. J Inorg Organomet Polym Mater. 2023;33(6):1437-52. doi:10.1007/s10904-023-02550-x
- Alhujaily M, Albukhaty S, Yusuf M, Mohammed MKA, Sulaiman GM, Al-Karagoly H, et al. Recent advances in plant-mediated zinc oxide nanoparticles with their significant biomedical properties. Bioengineering (Basel). 2022;9(10):541. doi:10.3390/bioengineering9100541
- Gharpure S, Ankamwar B. Synthesis and antimicrobial properties of zinc oxide nanoparticles. J Nanosci Nanotechnol. 2020;20(10):5977-96. doi:10.1166/jnn.2020.18707
- Blinov AV, Kachanov MD, Gvozdenko AA, Nagdalian AA, Blinova AA, Rekhman ZA, et al. Synthesis and characterization of zinc oxide nanoparticles stabilized with biopolymers for application in wound-healing mixed gels. Gels. 2023;9(1):57. doi:10.3390/gels9010057
- Czyżowska A, Barbasz A. A review: Zinc oxide nanoparticles - friends or enemies? Int J Environ Health Res. 2022;32(4):885-901. doi:10.1080/09603123.2020.1805415
- Murali M, Kalegowda N, Gowtham HG, Ansari MA, Alomary MN, Alghamdi S, et al. Plant-Mediated zinc oxide nanoparticles: Advances in the new millennium towards understanding their therapeutic role in biomedical applications. Pharmaceutics. 2021;13(10):1662. doi:10.3390/pharmaceutics13101662
- Asif N, Amir M, Fatma T. Recent advances in the synthesis, characterization and biomedical applications of zinc oxide nanoparticles. Bioprocess Biosyst Eng. 2023;46(10):1377-98. doi:10.1007/s00449-023-02886-1
- Jin SE, Jin HE. Synthesis, characterization, and three-dimensional structure generation of zinc oxide-based nanomedicine for biomedical applications. Pharmaceutics. 2019;11(11):575. doi:10.3390/pharmaceutics11110575
- Petetta F, Ciccocioppo R. Public perception of laboratory animal testing: Historical, philosophical, and ethical view. Addict Biol. 2021;26(6):e12991. doi:10.1111/adb.12991
- Xia W, Huang ZJ, Shi N, Feng YW, Tang AZ. Dosage selection and effect evaluation of sodium pentobarbital in tree shrew anesthesia. Lab Anim. 2023;57(3):283-92. doi:10.1177/00236772221146419
- Lee IO, Jeong YS. Effects of different concentrations of formalin on paw edema and pain behaviors in rats. J Korean Med Sci. 2002;17(1):81-5. doi:10.3346/jkms.2002.17.1.81
- He B, Nan G. Pulmonary edema and hemorrhage after acute spinal cord injury in rats. Spine J. 2016;16(4):547-51. doi:10.1016/j.spinee.2015.11.065
- Appelt P, Gabriel P, Bölter C, Fiedler N, Schierle K, Salameh A, et al. Left ventricular depression and pulmonary edema in rats after short-term normobaric hypoxia: Effects of adrenergic blockade and reduced fluid load. Pflugers Arch. 2021;473(11):1723-35. doi:10.1007/s00424-021-02618-y
- Lye TH, Roshankhah R, Karbalaeisadegh Y, Montgomery SA, Egan TM, Muller M, et al. In vivo assessment of pulmonary fibrosis and edema in rodents using the backscatter coefficient and envelope statistics. J Acoust Soc Am. 2021;150(1):183. doi:10.1121/10.0005481
- Kalisvaart ACJ, Abrahart AH, Coney AT, Gu S, Colbourne F. Intracranial pressure dysfunction following severe intracerebral hemorrhage in middle-aged rats. Transl Stroke Res. 2023;14(6):970-86. doi:10.1007/s12975-022-01102-8
- Rzhepakovsky I, Piskov S, Avanesyan S, Shakhbanov M, Sizonenko M, Timchenko L, et al. High-performance microcomputing tomography of chick embryo in the early stages of embryogenesis. Appl Sci. 2023;13(19):10642. doi:10.3390/app131910642
- Nurfahri R, Wahyuni I, Nurwasis N, Legowo D, Dhiyantari NPAR, Cinthiadewi MDGA. Expression of Bax, Bcl-2, and Bax/Bcl-2 ratio of Rattus norvegicus lens epithelial cells as a new approach to compare the protective effects of anti-UV-B glasses and anti-UV-B contact lenses from UV-B radiation: True experimental study in animal models. J Med Pharm Chem Res. 2024;6(8):1237-47. doi:10.48309/jmpcr.2024.441731.1120
- Li W, Zhang G, Wei X. Lidocaine-loaded reduced graphene oxide hydrogel for prolongation of effects of local anesthesia: In vitro and in vivo analyses. J Biomater Appl. 2021;35(8):1034-42. doi:10.1177/0885328220988462
- Huss MK, Felt SA, Pacharinsak C. Influence of pain and analgesia on orthopedic and wound-healing models in rats and mice. Comp Med. 2019;69(6):535-45. doi:10.30802/AALAS-CM-19-000013
- Zafar S, Ashraf MM, Ali A, Aslam N, Ashraf A, Zafar S, et al. Effect of caffeine on the anti-clotting activity of warfarin in healthy male albino rabbits. Pak J Pharm Sci. 2018;31(2(Suppl.)):611-6.
- El-Bahr SM, Shousha S, Albokhadaim I, Shehab A, Khattab W, Ahmed-Farid O, et al. Impact of dietary zinc oxide nanoparticles on selected serum biomarkers, lipid peroxidation and tissue gene expression of antioxidant enzymes and cytokines in Japanese quail. BMC Vet Res. 2020;16(1):349. doi:10.1186/s12917-020-02482-5
- Erfani Majd N, Tabandeh MR, Hosseinifar S, Rahimi Zarneh S. Chemical and green ZnO nanoparticles ameliorated adverse effects of cisplatin on histological structure, antioxidant defense system and neurotrophins expression in rat hippocampus. J Chem Neuroanat. 2021;116:101990. doi:10.1016/j.jchemneu.2021.101990
- Yi C, Yu Z, Ren Q, Liu X, Wang Y, Sun X, et al. Nanoscale ZnO-based photosensitizers for photodynamic therapy. Photodiagnosis Photodyn Ther. 2020;30:101694. doi:10.1016/j.pdpdt.2020.101694
- Alavi M, Nokhodchi A. An overview of antimicrobial and wound healing properties of ZnO nano biofilms, hydrogels, and bionanocomposites based on cellulose, chitosan, and alginate polymers. Carbohydr Polym. 2020;227:115349. doi:10.1016/j.carbpol.2019.115349
- Dukhinova MS, Prilepskii AY, Shtil AA, Vinogradov VV. Metal oxide nanoparticles in therapeutic regulation of macrophage functions. Nanomaterials (Basel). 2019;9(11):1631. doi:10.3390/nano9111631
- Arslan K, Karahan O, Okuş A, Unlü Y, Eryılmaz MA, Ay S, et al. Comparison of topical zinc oxide and silver sulfadiazine in burn wounds: An experimental study. Ulus Travma Acil Cerrahi Derg. 2012;18(5):376-83. doi:10.5505/tjtes.2012.45381
- Melkumyan VA, Kurbanova DA, Magomedgadzhieva RS, Khidiryan MV, Eremin SA, Stepanenko VS, et al. Assessment of wound-healing activity of zinc oxide nanoparticles. J Adv Pharm Educ Res. 2024;14(1):73-6. doi:10.51847/0SQk1Cmx1X
- Elhabal SF, Abdelaal N, Saeed Al-Zuhairy SAK, Elrefai MFM, Elsaid Hamdan AM, Khalifa MM, et al. Green synthesis of zinc oxide nanoparticles from Althaea officinalis flower extract coated with chitosan for potential healing effects on diabetic wounds by inhibiting TNF-α and IL-6/IL-1β signaling pathways. Int J Nanomedicine. 2024;19:3045-70. doi:10.2147/IJN.S455270
- Verevkina M, Goncharov V, Nesmeyanov E, Kamalova O, Baklanov I, Pokhilko A, et al. Application of the Se NPs-Chitosan molecular complex for the correction of selenium deficiency in rats model. Potravinarstvo Slovak J Food Sci. 2023;17:455-66. doi:10.5219/1871
- Musaev K, Edelbieva M, Askhabova F, Dudko N, Daudova M, Sultanova K, et al. Harnessing the power of a zinc oxide nanoparticles against viral pathology of the cornea: In vitro assessment. J Med Pharm Chem Res. 2024;7(1):64-73. doi:10.48309/jmpcr.2025.455914.1216
- Noor S, Al-Shamari A. High photocatalytic performance of ZnO and ZnO/CdS nanostructures against reactive blue 4 dye. J Med Pharm Chem Res. 2023;5(9):776-93.
- Vornic I, Pop O, Pascalau A, Andreescu G, Beiusan C, Manole F, et al. Assessing the role of Bcl-2 and p53 in apoptotic mechanisms in spontaneous abortions. Pharmacophore. 2024;15(2):1-6. doi:10.51847/CO2qttSgIN
- Cachón-Rodríguez G, Blanco-González A, Prado-Román C, Del-Castillo-Feito C. Studying the pattern of employee loyalty based on social capital and sustainable human resource management. J Organ Behav Res. 2024;9(2):1-11. doi:10.51847/fN33v3jKBU
- Liu J, Cheng X, Zhang Y, Wang X, Zou Q, Fu L. Investigating the effectiveness of modified clinoptilolite zeolite on nitrate removal from aqueous solution. J Biochem Technol. 2024;15(3):8-14. doi:10.51847/Hob35EiM0b
- Afrand Khalil Abad Z, Dehbokri N, Heydari Z, Karimi P, Siadat SH, Pakmehr SA, et al. The role of oxidative stress in the occurrence of neurological diseases and cancer. Clin Cancer Investig J. 2024;13(4):1-6. doi:10.51847/XQOJjb4ziu
- Shinde AA, Tayade AY, Bajolge R, Khyade VB. Efficiency of the zingiberene for the qualitative silk. Entomol Appl Sci Lett. 2023;10(1):1-10. doi:10.51847/4hW3o5bgkY
- Padma KR, Don KR, Anjum MR, Sindhu GS, Sankari M. Application of green energy technology for environmental sustainability. World J Environ Biosci. 2023;12(4):1-7. doi:10.51847/bAMKAPPZGe
- Domatskiy VN, Sivkova EI. Ixodes ticks – carriers of pathogens of vector-borne infections. Int J Pharm Res Allied Sci. 2024;13(1):74-82. doi:10.51847/DuluVQOxsp