Archive \ Volume.15 2024 Issue 4
Late Lymphocytopenia, Neutropenia, and Thrombocytopenia Following Axicabtagene Ciloleucel and Tisagenlecleucel CAR-T Cell Therapy: A Retrospective Study
Abstract
This study aimed to provide a 2024 update on cumulative survival (CS) and the incidence of lymphocytopenia, neutropenia, and thrombocytopenia, following axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) CAR-T cell therapies. It reviewed 56 consecutive patients who received adoptive CAR-T cell therapy for diffuse large B cell lymphoma, from 2019 to March 2024. Group A included 34 patients treated with axi-cel (Yescarta®), and Group B included 22 patients treated with tisa-cel (Kymriah®). It was estimated the cumulative survival (CS), by the Kaplan-Meyer method, and the occurrence of late cytopenias beyond day 60 (“late”), according to the most recent blood samples collected following the established protocol, the grading is done based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events.
The cohort (n=56) was composed of 49% female, 55 (9) years old, weight of 75 (23) kg, and height of 161(18) cm. 50.7% patients on axi-cel (Group A), and 32.8% on tisa-cel (Group B). The CS for axi-cel treated 65.5% and for 56.5% for tisa-cel and toxicity showed that overall lymphopenia, neutropenia, and thrombocytopenia were 21.4%, 5.3%, and 12.5%, respectively. The average disease-free period until the end of data collection (March 2024) was 19 (14) months, and the average time to death from any cause was 10 (9) months. The findings from our study suggest that the development of late cytopenia after CAR T-cell therapy is uncommon and may occur through various mechanisms in susceptible patients.
How to cite:
Download Citation
INTRODUCTION
Among other lymphoproliferative diseases, chimeric antigen receptor T-cell (CAR-T) therapy targeting the CD19 antigen is proven to be a rather successful treatment for diffuse large B cell lymphoma (DLBCL), therefore giving fresh hope for patients with limited therapeutic alternatives [1], however, the major influence of cytopenias on non-relapse mortality and morbidity is attracting more and more interest [2, 3].
Because of this, estimating real-world cumulative survival and the incidence of cytopenia, particularly late-onset cases occurring 60 days post-treatment, remains a formidable challenge [4, 5].
Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel), two of the approved therapies in Spain, may exhibit varying propensities for different types of cytotoxicity. Collecting information on Grade ≥4 cytopenias, and their impact on survival is particularly important and essential for optimizing treatment and improving patient outcomes [6, 7].
In this study, we aimed to provide a 2024 update on cumulative survival (CS) and the prevalence of late (beyond day 60 after CAR T infusion) lymphocytopenia, neutropenia, and thrombocytopenia following axi-cel and tisa-cel CAR-T cell therapies [8, 9].
MATERIALS AND METHODS
A cohort of consecutive patients from different autonomous CAR-T hospitals in Aragon, Spain, who had adoptive CAR-T cell treatment were studied from 2019 to March 2024.
The sample was composed of a real-world cohort of 56 patients treated with CAR-T cells targeting the CD19 antigen for diffuse large B-cell lymphoma (DLBCL) in the third line of treatment. These patients were divided into two groups: Group A included 34 patients treated with axi-cel (Yescarta®), and Group B included 22 patients treated with tisa-cel (Kymriah®).
We estimate the cumulative survival, by the Kaplan-Meyer (KM) method, and assessed the occurrence of late lympho-, neutro-, and thrombo-cytopenia, categorizing these cytopenias based on their timing and persistence beyond day 60 (“late”) [10, 11], using the most current blood samples taken from the research participants per the protocol, as part of tests that are considered clinically essential, and according to the grading system established by the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events [12]. As a result, a platelet count below 50 G/L was considered severe thrombocytopenia, a neutropenia count below 500/μL was considered profound, and a lymphocyte count between 200 and 499/µL was considered severe lymphopenia, with a count below 200/µL being considered very severe lymphopenia [13, 14].
Data was gathered from the hospital's lab and supported by electronic prescription recording systems. Survival estimates were evaluated using KM´s method, which estimates the proportion of patients still alive over time while accounting for subjects censored before the event of interest. Kaplan-Meier estimates for the cumulative survival of patients over 50 months was determined starting from the moment of CAR-T injection. In addition, the log-rank test was employed to compare the survival distributions of the two samples, namely groups A and B.
Finally, we also calculated the percentage of severe (grade ≥º4) lymphocytopenia, neutropenia, and thrombocytopenia, based on the latest hematological analysis (before death or the most recent data in patients that were alive), and compared these percentages using Fisher's exact test.
As the study was retrospective and non-experimental, with pooled data obtained from secondary sources and conducted in Spanish public hospitals, review by an ethical committee was not necessary. However, this research adhered to the ethical guidelines outlined in the 1975 Declaration of Helsinki, updated in 2013.
RESULTS AND DISCUSSION
Of the 56 patients, the average (± standard deviation, SD) follow-up period per patient was 16 (13) months until the end of the study. The cohort was 49% female, with an age of 55 (9) years, weight of 75 (23) kg, and height of 161 (18) cm. 34 (50.7%) patients were infused with axi-cel (Group A), and 22 (32.8%) were treated with tisa-cel (Group B), for DLBCL.
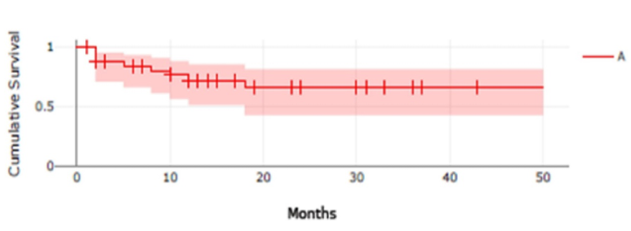
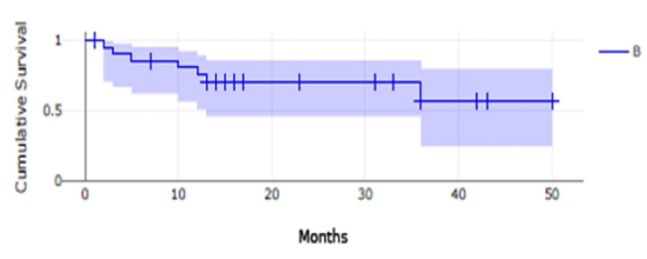
The estimated CS for the 34 axi-cel treated patients was 65.5% (n=34; events = 9, censored=25; survival rate = 0.6554, SD=0.09986, CI 95%= 0.4246-0.8118); and for 22 tisa-cel treated patients, the estimated CS was 56.5% (n=22; events=7, censored=15; survival rate = 0.5647, SD=0.15, CI95%=0.2415-0.7947).
|
|
|
a) Survival Function (St) – with confidence interval |
|
|
|
b) Survival Function (St) – with confidence interval |
|
Figure 1. Cumulative Survival Kaplan-Meier Curves |
This figure presents Kaplan-Meier cumulative survival curves for 34 subjects who received axi-cel (Yescarta®) CAR T cell therapy (A) and 22 subjects who received tisa-cel (Kymriah®) CAR T cell therapy (B). The shaded regions indicate the 95% confidence intervals. The number of subjects at risk is presented at 1-month intervals, and vertical tick marks indicate time points with censored data.
The log-rank test for the two groups, A and B, found no statistically significant differences between them (p-value = 0.996191; p(x≤χ²) = 0.00380938).
12 patients (21.4%) experienced severe lymphocytopenia (n=7, 58.3%, with axi-cel and n=5, 41.7%, with tisa-cel), with 7 (58.3%) of these cases being very severe, and 9 (75%) of these patients died; 3 patients (5.3%) had profound neutropenia (all with tisa-cel and all deceased); and 7 patients (12.5%) experienced thrombocytopenia (n=5, 8.9%, with axi-cel, and of these, 4, 80%, died).
The average disease-free period until the end of data collection (March 2024) was 19 (14) months, and the average time to death from any cause was 10 (9) months.
An overview of toxicity according to the NCI showed that the overall distribution of life-threatening (≥ º4) lymphopenia, neutropenia, and thrombocytopenia was 21.4%, 5.3%, and 12.5%, respectively. There were differences, although not statistically significant, between groups A and B, regarding the percentage of cytopenia and/or CS.
CD19-targeted chimeric antigen receptor (CAR) T-cell therapy has revolutionized the management of certain recurrent or resistant lymphoproliferative diseases, such as B-cell lymphomas [15].
In our study, CS data align closely with published literature, 65.5%, for axi-cel, and 56.5%, for tisa-cel, both consistent with bibliographic data of 67%-74% [16].
On the other hand, late cytopenias, occurring after CAR-T infusion, are not completely understood and may be related to prior bone marrow infiltration, previous lines of chemotherapy and/or radiation therapy, variations in the costimulatory domains that drive CAR-T cell expansion and persistence, and disease-related and CAR-T infusion factors all contribute to bone marrow dysfunction and chronic cytopenias following CAR-T therapy [17, 18].
The variation in cytokine release risk across CAR-T products may be associated with the design of the CAR construct and its impact on cytopenias [19]. In fact, we know that although the exons of the cluster of differentiation (CD) markers CD45 and CD19 are different, CD19 CAR-T cells are prepared to target the body's own cells. In this case, B cells contain both CD19 and CD45 (the latter is also present throughout the lymphoid series).
Due to the above, we can suggest that cross-reactivity may occur between B cells expressing CD19 and CD45, targeted by both axi-cel and tisa-cel, and other lymphoid series cells expressing CD45 (e.g., B and T lymphocytes, neutrophils, and platelets), inducing autoantigens related to CD45 or another common antigen and resulting in some cytopenias.
In summary, despite the remarkable therapeutic efficacy of commercial CAR-T cell therapies, concerns over toxicities remain. Recent reports have described concerns over vector integration causing the emergence of antigens related to CD45, which could generate cross-reactivity between cells expressing both CD19 and CD45 or another common marker, present in myeloid and lymphoid hematopoietic cells, leading to pancytopenia [16].
Important constraints of this study include its retrospective design and the inclusion of late ICAHT grades (beyond day +60) which were not accessible for the majority of cases due to loss of follow-up and/or insufficient high-quality data after day 60. Although this study included patients treated with CAR-T cells in a real-world scenario, there was significant variation in terms of underlying patient characteristics, treatment settings, and toxicity management. In addition, we did not examine variables such as CAR T-cell product, kind of malignancy, and other factors that potentially impact the occurrence of late cytopenias and the rate of survival due to the limited size of the group.
CONCLUSION
The findings from our study suggest that the development of late cytopenia after CAR T-cell treatment is uncommon and may occur through various mechanisms in susceptible patients.
ACKNOWLEDGMENTS: None
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: None
References
- June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64-73.
- Lemoine J, Bachy E, Cartron G, Beauvais D, Gastinne T, Rubio MT, et al. Causes and risk factors of early and late non-relapse mortality after CD19 CAR T-cell therapy for diffuse large B-cell lymphoma (DLBCL): A Lysa study from the Descar-T registry. Blood. 2022;140(Suppl 1):1859-61.
- Johnsrud A, Craig J, Baird J, Spiegel J, Muffly L, Zehnder J, et al. Incidence and risk factors associated with bleeding and thrombosis following chimeric antigen receptor T-cell therapy. Blood Adv. 2021;5(21):4465-75.
- Sofiah M, Lestari K, Barliana M, Parwati I, Halimah E. bla SHV-12 gene detection from Klebsiella pneumoniae producing Extended-Spectrum β-Lactamase using amplification-refractory mutation system method. J Adv Pharm Educ Res. 2022;12(2-2022):76-83.
- Salama NM, El-Rokh ES, Hashem G, Mowafy HH, Elsissy MH, Labib DA. Clopidogrel versus ticagrelor in elective percutaneous coronary intervention. J Adv Pharm Educ Res. 2022;12(2-2022):30-7.
- Florina MG, Mariana G, Csaba N, Gratiela VL. The interdependence between diet, microbiome, and human body health-A systemic review. Pharmacophore. 2022;13(2):1-6.
- Kumar R, Singh G. Substituted benzimidazoles as antibacterial and antifungal agents: A review. Pharmacophore. 2022;13(2-2022):41-55.
- Nabavi SS, Gholizadeh B. Evaluation of the quality of life of the patients with heart failure in Ahvaz teaching hospitals. Entomol Appl Sci Lett. 2022;9(1-2022):26-30.
- Canassa VF, Baldin EL. Nymphal performance and fecundity of melanaphis sacchari (Zehntner)(Hemiptera: Aphididae) in different sorghum genotypes. Entomol Appl Sci Lett. 2022;9(2-2022):1-0.
- Taneja A, Jain T. CAR-T-OPENIA: Chimeric antigen receptor T-cell therapy-associated cytopenias. eJHaem. 2021;3(Suppl 1):32-8.
- Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR-T cell therapy. Blood. 2023;141(20):2460-9.
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- Gaikwad SS, Choudhari VP. Efficacy and safety of combination therapy of zinc and silver oxide nanoparticles in streptozotocin-induced diabetic rats. Int J Pharm Res Allied Sci. 2022;11(3-2022):1-0.
- Nizkii S, Kodirova G, Kubankova G. Lysine-an absolutely essential amino acid in soybean proteins from the Russian selection. Int J Pharm Res Allied Sci. 2022;11(1-2022):51-4.
- Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;77(26):2531-44.
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56.
- Kitamura W, Asada N, Naoi Y, Abe M, Fujiwara H, Ennishi D, et al. Bone marrow microenvironment disruption and sustained inflammation with prolonged haematologic toxicity after CAR T-cell therapy. Br J Haematol. 2023;202(2):294-307.
- Zhou J, Zhang Y, Shan M, Zong X, Geng H, Li J, et al. Cytopenia after chimeric antigen receptor T cell immunotherapy in relapsed or refractory lymphoma. Front Immunol. 2022;13:997589.
- Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640-54.