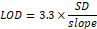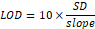Archive \ Volume.15 2024 Issue 4
Novel Analytical Method for Combined Dapagliflozin and Vildagliptin in Bulk and Pharmaceutical Dosage Form Using HPLC
Abstract
A straightforward, exact, and reliable method has been devised to measure Dapagliflozin and Vildagliptin in bulk or combined tablet dose form concurrently by RP-HPLC. The chromatographic conditions used were an Agilent C18 column (150 x 4.6 mm, 5 mm) with a gradient mode of Orthophosphoric acid: Acetonitrile (pH5) as the mobile phase and a flow rate of 0.7 ml/min. The maximum absorbance was determined to be 224nm by UV Spectrophotometer. Dapagliflozin and Vildagliptin Retention times were found to be 3.5 and 2.1 minutes, respectively. The established method was validated in accordance with ICH Q2 (R1) methods. The Linearity was determined from 0.032:0.32 to 10:100µg/ml. The Percentage RSD was found to be less than 2% which was found to be within the limits. The Accuracy was found to be between 98 to 102% which was found to be within the limits. The robustness data was done by increasing and decreasing the flow rate between 0.6ml and 0.7ml. For the RP-HPLC technique, the concentration range from micrograms/ml yields linear responses.
How to cite:
Download Citation
INTRODUCTION
Two anti-diabetic drugs, dapagliflozin and vildagliptin, are combined. Dapagliflozin lowers the level of glucose in the blood as well as enhances urine glucose excretion [1]. By increasing insulin levels and lowering glucagon levels (the hormone that raises blood glucose levels), vildagliptin lowers the quantity of glucose generated by the liver [2]. Consequently, a method for measuring Dapagliflozin and Vildagliptin in bulk and combination tablet dose forms must be needed. Dapagliflozin and Vildagliptin were determined concurrently in bulk and composite tablet dose forms using an RP-HPLC technique [3]. Drugs Profile is given in the below Table 1.
|
Table 1. Drug Profile of Dapagliflozin and Vildagliptin |
||
|
Parameters |
Dapagliflozin |
Vildagliptin |
|
Colour |
The White crystalline powder |
The White crystalline powder |
|
Molecular formula |
C21H25ClO6 [4] |
C17H25N3O2 |
|
Molecular weight |
408.9 [5] |
303.4 |
|
Category |
sodium-glucose co-transporter 2 (SGLT2) inhibitors [6] |
Oral anti-hyperglycemic agent of the dipeptidyl peptidase-4 (DPP-4) inhibitor [7] |
|
USFDA Approved |
Jan 8, 2014 [8] |
Jan 20,2010 [9] |
|
USFDA(dapa+Vilda) |
DAPAGLIFLOZIN AND VILDAGLIPTIN |
Jan 8, 2022 [10] |
MATERIALS AND METHODS
Equipment and Apparatus
Shimadzu (LC-20AD) equipment and a computerized analytical balance, including an ultrasonic water bath were used along with volumetric flasks, measuring cylinders, pipettes and beakers.
Chemicals and Reagents
Dapagliflozin and Vildagliptin standard were given out as free samples by the pharmaceutical company and purchased the written prescription for Glyduo (Dapagliflozin as well as Vildagliptin 10:100) from a pharmacy store. All of the experiment's ingredients were of the HPLC grade.
Dapagliflozin Standard Preparation
Ten mg of dapagliflozin was precisely weighed and placed in a 10 ml volumetric flask. To aid in the medication's dissolution, add a tiny bit of water. To reach 1000 μg/mL, add double-distilled water up to the mark. First, pipette 1 mL of 1000 μg/mL into a ten-milliliter volumetric flask. Then, diluent it to the proper concentration, 100μg/mL as needed.
Vildagliptin Standard Preparation
Vildagliptin10 mg was precisely weighed and administered into a Volumetric flask of 10ml. Add a little water to help the medicine dissolve. Next, add water to reach the 1000 parts per million level. Fill a 10 mL volumetric flask with 1 mL of a 1000μg/mL solution by pipetting it, and then dilute it to the required concentration of 100μg/mL.
Selecting a Wavelength
To determine the isosbestic point, the UV-Spectrophotometer was used to scan both of the standard 10 ppm concentrations from 200 to 400 nm in overlay mode for selecting isobestic point.
Chromatographic Conditions
Column: Agilent
Injection volume :20μl
Detector: UV(224nm)
Mobile phase: OPA:ACN
Pump mode: Gradient
Flow rate: 0.7ml
Run time: Vildagliptin 2.1, Dapagliflozin 3.5
Run time: 10min.
Validation Parameters
Specificity
To determine the specificity, any disruption in the optimized approach can be examined. We shouldn't get interfering peaks in either placebo or blank samples during the retention durations of these medications using this method. Therefore, it was claimed that this strategy was specific. The injection of the blank solutions was done to achieve the specificity.
Linearity
Standard Dapagliflozin Preparation
A precise weight of 10 mg of dapagliflozin was taken in a volumetric flask with a capacity of 10 ml. Water in small amounts is added to dissolve the medication. Then add water to the mark to get 1000 µg/ml of makeup. To get a concentration of 100µg/ml, pipette out 1 ml from 1000µg/ml and transfer it into a 10 ml volumetric flask. Mark the makeup with diluent. To get a 10µg/ml concentration, pipette out 1 ml from the 100µg/ml and transfer it into a 10 ml volumetric flask. Mark the makeup with diluent.
Standard Vildagliptin Preparation
Vildagliptin (10 mg) was precisely weighed and administered into a 10-milliliter volumetric flask. Water in small amounts is added to dissolve the medication. Then add water to the mark to get 1000 µg/ml of makeup. To get a concentration of 100µg/ml, pipette out 1 ml from 1000µg/ml and transfer it into a 10 ml volumetric flask. Mark the makeup with diluent. Concentrations for linearity were prepared as given in Table 2.
|
Table 2. Preparation of different concentrations for linearity |
|||
|
S.No. |
Concentration |
Dapagliflozin |
VILDAGLIPTIN |
|
1 |
0.032:0.32 |
0.032 |
0.32 |
|
2 |
0.05:0.53 |
0.05 |
0.53 |
|
3 |
0.16:1.6 |
0.16ml |
1.6ml |
|
4 |
0.33:3.33 |
0.33ml |
3.33ml |
|
5 |
1.1:11 |
1.1ml |
11ml |
|
6 |
1.4:14 |
1.4ml |
14ml |
|
7 |
1.7:17 |
1.7ml |
17ml |
|
8 |
2.0:20 |
2.0ml |
20ml |
Precision
Precision is a term used to describe how closely two measurements taken under certain conditions from various portions of the same homogenous sample coincide [11].
Intraday Precision: It is defined as accuracy maintained under the same circumstances throughout a short period. Throughout the intraday precision, six injections with a ratio of 0.16:1.6 were made [12].
Inter-Day Precision: It is performed within the lab variations, which include various days, different instruments, and different analysts. A precise injection of 0.16:1.6 was administered six times over the day [13].
Accuracy
The degree of agreement between the method's calculated value and the true value of the analytes in the sample is known as accuracy [14].
Sample Solution Preparation
Sample solution of Combined dosage form was prepared by taking weight equivalent to 10mg in a 10ml volumetric flask and dilute it with respective solvents to obtain 1000µg/ml. Then make 100µg/ml from this 1000µg/ml.Take 0.25:2.5 as the sample concentration
- By adding three concentrations of standard solution i.e. 50,100,150. to sample solution, the accuracy was ascertained.
- Standard quantity equal to 50%, 100%, 150% is to be added in sample 2ml of 0.25:2.5ppm sample was spiked to 2ml of 0.5:5ppm standard solution,
- 2ml of 0.25:2.5ppm of sample was spiked to 1:10ppm of standard solution,
- 2ml of 0.25:2.5ppm sample solution was spiked to 2ml of 1.5:15ppm of standard solution.
Absorbance was measured three times at 224nm. The % recovery was determined.
Detection Limit and Quantification Limit
The smallest quantity of analyte that can be identified is known as the detection limit. The lowest amount of analyte that can be detected is known as the quantitation limit. computed with the help of the formula below [15, 16].
|
|
(1) |
|
|
(2) |
Robustness
Dapagliflozin and Vildagliptin (1:10) were analyzed by varying the flow rate while maintaining the same parameters. The solution's Responce was measured at flow rates of 0.7 and 0.8 millilitres per minute.
RESULTS AND DISCUSSION
Linearity
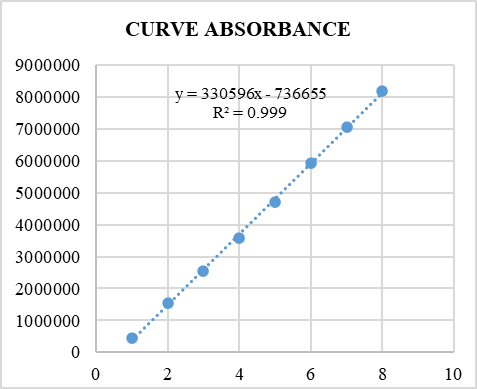
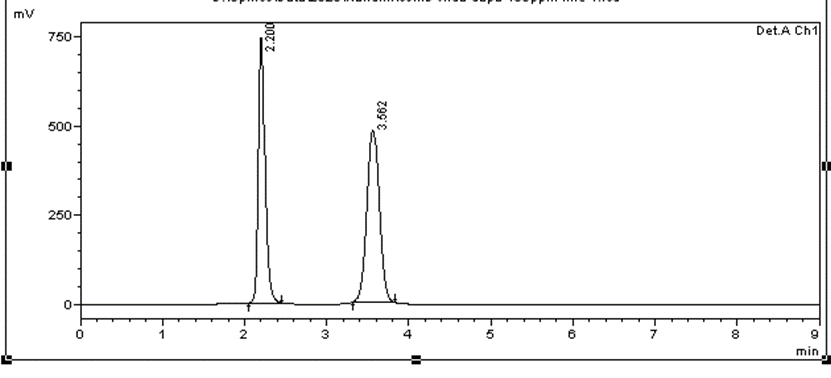
Linearity data of combined dosage form of Dapagliflozin and vildagliptin is given in Table 3. Calibration plot of combined dosage form is given in Figure 1 and HPLC chromatogram for linearity levels 0.032:0.32µg/ml and 10:100µg/ml given in Figures 2 and 3.
|
Table 3. Results of Linearity of the combined dosage form (Dapa+Vilda) |
|||||
|
Concentration (µg/ml) |
Area |
Ret. Time (Dapa) |
Ret. Time (Vilda) |
Resolution (Dapa) |
Resolution (Vilda) |
|
0.032:0.32 |
46092 |
3.587 |
2.201 |
7.458 |
0.00 |
|
0.16:1.6 |
15322042 |
3.552 |
2.191 |
5.983 |
0.00 |
|
1:10 |
2237126 |
3.570 |
2.200 |
5.918 |
0.000 |
|
2:20 |
3589302 |
3.552 |
2.188 |
5.787 |
0.000 |
|
4:40 |
4696010 |
3.564 |
2.199 |
5.821 |
0.000 |
|
6:60 |
5943812 |
3.564 |
2.184 |
5.741 |
0.000 |
|
8:80 |
7063314 |
3.563 |
2.189 |
5.639 |
0.000 |
|
10:100 |
8195683 |
3.562 |
2.200 |
6.140 |
0.000 |
|
|
|
Figure 1. Calibration plot of combined dosage form |
|
|
|
Figure 2. Chromatogram for linearity level 0.032:0.32µg/ml |
|
|
|
Figure 3. Chromatogram for linearity level of 10:100µg/ml |
Precision
Intraday Precision
Intraday precision is defined as accuracy maintained within the same conditions for a short period. The intraday precision involved six injections at a ratio of 1:10. Results of Intraday precision of Dapagliflozin and Vildagliptin were found to be within the limits. For analyst-1 peak areas, standard deviation was found to be 2876317.397 and %RSD was found to be 1.336325982%. For analyst-2 peak areas, standard deviation was found to be 34411.11555 and %RSD was found to be 1.526183919%.
Inter-Day Precision
This is done within laboratory variances, which include various days, various equipment, and different analysts. Throughout the day, six precise 1:10 injections were administered. Results of Interday precision of Dapagliflozin and Vildagliptin were found to be within the limits. For Day-1 peak areas, standard deviation was found to be 2876317.397 and %RSD was found to be 1.336325982%. For Day-2 peak areas, standard deviation was found to be 34411.11555 and %RSD was found to be 1.526183919 %.
Accuracy
Sample solution preparation involved weighing and transferring powder equal to ten milligrams of a medication known as Dapagliflozin as well as 100 milligrams of vildagliptin into a 100 ml volumetric flask filled with acetonitrile. The volume was then increased to the required amount with acetonitrile while shaking the flask intermittently (100 ppm). A membrane filter with a thickness of 0.45 μm was used to filter the final solution. Additionally, one millilitre of a clear filtrate was added to a volumetric flask with a volume of and diluted to the appropriate level.
By adding standard solution at three different concentrations—50, 100, and 150—to the sample solution, the accuracy was ascertained. Standard amounts of 50%, 100%, and 150% must be added to the sample. Two millilitres of the 10 parts per millilitre sample were mixed with two millilitres of the 0.5:5 standard solution, and two millilitres of the 10 parts per millilitre sample were mixed with one millilitre of the 1:10 standard solution. Accuracy data of Dapagliflozin and Vildagliptin were given in Table 4.
|
Table 4. Results of Accuracy data of Dapagliflozin and Vildagliptin |
||||
|
Accuracy Level |
Combined Drug Conc (ppm) |
Spiked Conc (ppm) |
% Recovery |
Mean %Recovery |
|
50% |
0.5: 5 (D+V) |
10 |
98. 85 % 97. 90 % 98. 75 % |
98.5% |
|
100% |
1: 10 (D+V ) |
10 |
99. 93% 99.59 % 98. 85% |
99.45% |
|
150% |
1.5:15 ( D+V) |
10 |
97.71 % 97.87 % 98.69 % |
98.09% |
Robustness
Vildagliptin (1:10) and dapagliflozin were analysed while maintaining the same parameters and varying the flow rate. The solution's response was measured at flow rates of 0.6 and 0.7 millilitres per minute and the % RSD was found to be within the limits.
CONCLUSION
Dapagliflozin and Vildagliptin bulk and mixed tablet dose formulations were examined. It was discovered that the percentage of medicines in concurrent medications was within the Indian Pharmacopoeia's limitations. All confirmation parameters were checked, and it was discovered to be within permissible bounds in accordance with IHQ2(R2) specifications. Consequently, with the suggested method, Dapagliflozin and Vildagliptin alone and in conjunction with additional medications may be calculated with an RP-HPLC. For the suggested HPLC grade Acetonitrile: Water (50:50) technique was applied. Agilent (150 mm x 4.6 mm, 5 microns) column, as mobile phase flow rate of 0.7 ml/min, and UV scanning of the eluents 224 nm detector in the system. In gradient mode, it was discovered that the retention times for Dapagliflozin and Vildagliptin were 3.5 and 2.1 minutes, respectively. Consequently, this suggested approach was discovered to be superior to previously published techniques.
ACKNOWLEDGMENTS: I want to acknowledge our beloved principal Prof. M. Sumakanth and Faculty of department of Pharmaceutical Analysis for giving me this opportunity to perform the research work.
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: None
References
- Borse LB, Wagh MS, Borse SL, Ahire SP, Vaishnav IS, Naphade VD, et al. RP-HPLC method development and validation for estimation of dapagliflozin in tablet formulation. J Pharm Negat Results. 2022:364-72.
- Gaikwad AV, Gawade AS, Hupparage Vrushabh B, Mantry S, Kale A, Kale J. Method development and validation of dapagliflozin by RP-HPLC. J Pharm Negat Results. 2022;13(06):4316-35.
- Dhara V, Hetvi C. Development and validation of UV spectroscopic method for simultaneous estimation of remogliflozin etabonate and vildagliptin in bulk and pharmaceutical dosage form. Asian J Pharm Anal. 2023;13(2):69-73.
- Kumari B, Khansili A. Analytical method development and validation of UV-visible Spectrophotometric method for the estimation of vildagliptin in gastric medium. Drug Res (Stuttg). 2020;70(9):417-23. doi:10.1055/a-1217-0296
- Chaphekar MM, Hamrapurkar PD. Development and validation of RP-HPLC assay method for vildagliptin using QbD approach and its application to forced degradation studies. Int J Pharm Sci Drug Res. 2016;8(03):157-65. doi:10.25004/ijpsdr.2016.080306
- Naveed S, Rehman H, Qamar F, Zainab S. Method development and validation of Vildagliptin using UV spectrophotometer. Int J Pharm Sci Res. 2014;5(10):714-7.
- Manasa S, Dhanalakshmi K, Reddy NG, Sreenivasa S. Method development and validation of dapagliflozin in API by RP-HPLC and UV-spectroscopy. Int J Pharm Sci Drug Res. 2014;6(3):250.
- Karuna PC, China E, Rao MB. Unique UV spectrophotometric method for reckoning of Dapagliflozin in bulk and pharmaceutical dosage forms. J Chem Pharm Res. 2015;7(9):45-9.
- Tripathi KD. Medical pharmacology. 6th edition, Jaypee brothersmedical publishers; 2009.
- Rang H, Dale M, Ritter JM, Moore PK. Pharmacology. 5th edition, Elsevier publication; 2006.
- Donepudi S, Achanta S. Simultaneous estimation of saxagliptin and dapagliflozin in human plasma by validated high performance liquid chromatography-ultraviolet method. Turk J Pharm Sci. 2019;16(2):227-33. doi:10.4274/tjps.galenos.2018.46547
- Tortora GJ, Derrickson B. Principle of anatomy and physiology. 11th edition, John Wiley and son’s publishers; 2006.
- Debata J, Kumar S, Jha SK, Khan A. A New RP-HPLC method development and validation of dapagliflozin in bulk and tablet dosage form. Int J Drug Dev Res. 2017;9(2):48-51.
- Pallavi K, Srinivasa Babu P, Kishore Babu G. Development and validation of UV spectrophotometric method and RP-HPLC method for estimation of capecitabine in bulk and tablet dosage forms. Int J Appl Pharm. 2016;8(3):24-9.
- Chandana M, DR. Rao M, Samrajyam B, Sarissha KSKD, Naga Premi VV. Analytical method development and validation of vildagliptin in pharmaceutical dosage form by RP-HPLC method. J Health Sci Nurs. 2016;1.
- Lokhande DP. Analytical method development and validation of teneligliptin by using RP-HPLC with ICH guidelines. Int J Trend Sci Res Dev. 2019;3(3):259-63.