Archive \ Volume.15 2024 Issue 4
Investigation of Wound Healing Properties of Aqueous Extracts of Caucasus Herbs at Diabetes Mellitus
Abstract
Today, diabetes mellitus is one of the most common pathologies that leads to severe systemic complications. In this article, we studied the regenerative processes of an infected wound in hyperglycemia using dosage forms based on plant raw materials Acacia nilotica, Cyperus rotundus, Trigonella foenumgraecum, and Cymbopogon proximus. A model of dexamethasone-induced hyperglycemia was experimentally reproduced, followed by the creation of a wound and double application of Streptococcus epidermidis-infected material from a patient with streptoderma to the wound area. During the study, we found that the use of aqueous emulsions containing Acacia nilotica, Trigonella foenumgraecum, Cyperus rotundus, and Cymbopogon proximus has a positive effect on dysmetabolic disorders that occur during experimental dexamethasone-induced hyperglycemia (activity of hepatic transaminases decreases, blood glucose levels normalize), peripheral blood composition is optimized (the level of erythrocytes and hemoglobin, leukopenia is leveled). Such shifts create a favorable background for the course of reparative processes against the background of dexamethasone-induced damage, which inhibits the course of regenerative processes.
How to cite:
Download Citation
INTRODUCTION
Today, diabetes mellitus is one of the most common pathologies that leads to severe systemic complications [1-3]. High titers of corticosteroids initiate a cascade of catabolic processes, which affect the deactivation of regeneration processes of both epithelial tissues and wound processes [4-6]. The corrective factor of dysmetabolic disorders caused by diabetes mellitus is a directed diet [7, 8]. In this case, the greatest positive effect is achieved by combining pharmacological and nutritional components (hypocholesterolemic, antioxidant, hypoglycemic, and immunomodulatory effects) [9-11].
Phytotherapy is a recognized treatment method in official and folk medicine [12-14]. The interest in this method of exposure is increasing due to its lower toxicity and greater commitment to use, especially in countries with traditionally developed folk medicine [15, 16]. Cymbopogon proximus, Acacia nilotica, Trigonella foenumgraecum, and Suregis rotundus have a wide range of pharmacological effects [17, 18]. Among them, the most valuable are antiatherogenic, antidiabetic, antianorexic, antioxidant, anti-carcinogenic, hypolipidemic, and anti-inflammatory effects [19-22].
It is worth noting that in patients with type 2 diabetes mellitus, as well as mild diabetes mellitus, phytotherapy can be used in the form of monotherapy, providing stabilization or regression of the disease [23, 24]. Prolonged use of herbal remedies for this disease improves the general well-being of patients, and their quality of life, in general, reduces hyperglycemia, which allows you to reduce doses of antidiabetic drugs and even sometimes do without them in the future [25, 26].
Therefore, this work aimed to study the regenerative processes of an infected wound in hyperglycemia using dosage forms based on Caucasus herbs: Acacia nilotica, Cyperus rotundus, Trigonella foenumgraecum, and Cymbopogon proximus.
MATERIALS AND METHODS
The study was performed on 35 nonlinear white rats of both sexes weighing 200-250 g. All animal manipulations were carried out by the Guidelines for the Maintenance and Use of Laboratory Animals in the Russian Federation.
Modeling of hyperglycemia was achieved by intramuscular administration of 0.2 mL of 4% dexamethasone solution for 4 days. The skin on the back was treated with depilatory cream, and cleaned, local anesthesia with 0.25% novocaine solution was previously performed. Then, a 2 cm razor incision (0.5 mm deep) was applied to the prepared zone [27]. A double application of Streptococcus epidermidis-infected material from a patient with streptoderma was performed in the wound area.
All experimental animals were randomly divided into 7 groups of 5 rats each: intact group (group 1), 1st control group where the wound was treated with vaseline (group 2), 2nd control group where the wound was treated with fusidine (group 3), Experimental group where the wound was treated with an emulsion containing C. proximus and rats received orally an aqueous extract of C. proximus (group 4), an experimental group with topical application of ointment based on C. rotundus and vaseline and oral administration of aqueous extract of C. rotundus (group 5), experimental group with oral administration of aqueous extract of A. nilotica and topical application of an ointment based on A. nilotica (group 6), an experimental group with oral administration of an aqueous extract of Tr. foenumgraecum and topical application of ointment based on Tr. foenumgraecum (group 7).
On the 5th day of the experiment, a visual assessment of local changes in the wound area was performed according to the following semi-quantitative criteria (on the plus side – compaction, roughness, pain on palpation, hyperemia, swelling, regeneration) [28, 29]. The severity of the signs was assessed on a scale where the maximum severity of the signs was estimated at 5 points, the absence of a sign – at 0 points [30, 31].
Blood was taken from the caudal vein, and stabilized with a 10% sodium citrate solution, the blood composition and biochemical composition of the blood were determined using analyzers PCE-90Vet (USA) and HUMASTAR 600 (Germany), respectively [32, 33]. On the 5th day, the animals were removed from the experiment in the chamber with ether for anesthesia.
Statistical data processing was performed using the STATISTICA 12 software. The critical level of significance in testing statistical hypotheses in this study was assumed to be 0.05. In the course of the presentation, statistical indicators are indicated by the following symbols: M is the sample average, m (SEM) is the error of the average, and p is the achieved level of significance at the value.
RESULTS AND DISCUSSION
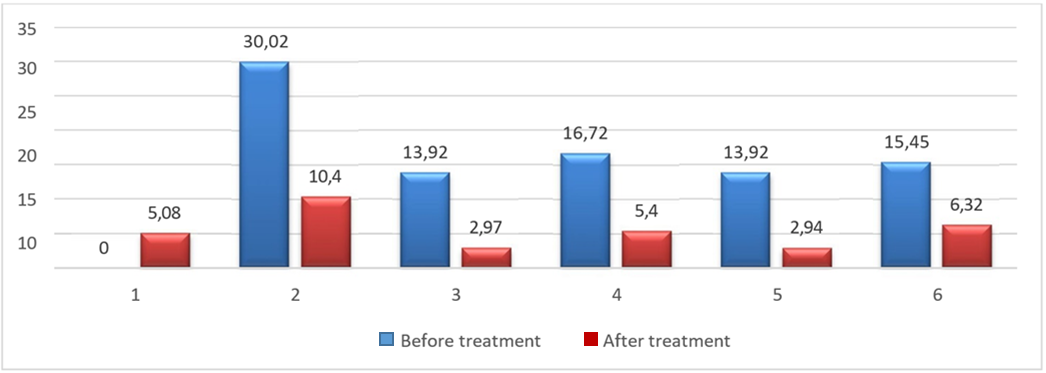
It was found that the administration of dexamethasone solution to rats at the rate of 800 µg/kg for 4 days leads to the development of hyperglycemia (Figure 1).
|
|
|
Figure 1. Dynamics of blood glucose levels in white rats with dexamethasone hyperglycemia and infected wounds during treatment with aqueous extracts (mmol/L): 1 - intact, 2 – dexamethasone solution, 3 – dexamethasone solution + aqueous extract of Cyperus rotundus, 4 – dexamethasone solution + aqueous extract of Cymbopogon proximus, 5 - dexamethasone solution + aqueous extract of Acacia nilotica, 6 - dexamethasone solution + aqueous extract of Trigonella foenumgraecum |
An increase in target glucose titers was recorded 6 times – up to 30.02 ± 5.16 mmol/L with an initial level of 5.08 ± 1.18 mmol/L, p<0.001. Oral administration of aqueous extracts from the 1st day of dexamethasone administration helped to correct glucose levels. The maximum hypoglycemic effect was recorded with oral administration of extracts of Acacia nilotica and Cyperus rotundus, showing that hypoglycemia occurred only 2.74 times, p<0.001. Oral administration of Trigonella foenumgraecum extracts showed an increase in glycemia by 304%, and Cymbopogon proximus by 329%, p<0.001.
After discontinuation of dexamethasone solution, the glucose titer in the group of animals without correction decreased by 65% and amounted to 10.4 ± 2.85 mmol/L, p<0.001, but remained almost 2 times higher than in intact animals, p<0.001. Persistent hyperglycemia persisted after dexamethasone administration. Oral administration of aqueous extracts containing the studied herbs and topical application of ointments had a pronounced hypoglycemic effect. This was most pronounced against the background of the introduction of Acacia nilotica and Cyperus rotundus. Trigonella foenumgraecum and Cymbopogon proximus reduced glucose level to 6.32 ± 0.58 mmol/L and 5.4 ± 0.79 mmol/L, respectively, p<0.01. Interestingly, fusidine was found to increase the activity of hepatic transaminases: ALT was increased by 341% and AST by 134%, p<0.01 (Table 1).
|
Table 1. Biochemical parameters of blood serum of white rats with hyperglycemia against the background of administration of dexamethasone solution with oral administration of aqueous extracts and local application of emulsions containing Acacia nilotica, Cymbopogon proximus, Trigonella foenumgraecum, Cyperus rotundus (M ± m) |
|||||||
|
Index |
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
Group 6 |
Group 7 |
|
ALT, units/L |
46.6±9.22 |
41±10.2* |
209.5±3.69* |
128.5±25.1#+* |
207±73.07+* |
138±32.1#+* |
135±37.7#+* |
|
Albumins, g/L |
29.61±0.8 |
29.7±2.98* |
31.5±3.10* |
34±2.16+* |
32.5±4.04 |
32.25±1.7 |
29.5±3.69 |
|
AST, units/L |
137.1±15.6 |
147.1±20 |
302.5±7.54*+* |
271.25±20#+* |
124±20.01# |
208.7±7.6#+* |
245.2±67.6+* |
|
Creatinine, mg/dL |
0.04±0.004 |
0.04±0.005* |
0.05±0.005 |
0.04±0.005 |
0.053±0.003 |
0.045±0.01 |
0.051±0.009 |
|
Urea, mmol/L |
4.52±0.51 |
5.2±0.87 |
6.15±0.33* # |
8.6±1.46#+* |
12.37±4.0#+* |
10.1±2.2#+* |
11.35±1.71#+* |
|
Total protein, g/L |
55.33±4.92 |
64±5.61 |
60.5±5.80 |
63±6.78 +* |
70±7.3 |
64.7±3.30 |
58±5.22 |
|
Total cholesterol, mmol/L |
0.55±0.08 |
1.3±0.27* |
1.47±0.17 * |
1.375±0.27 |
2.25±0.88+* |
1.7±0.29 +* |
2.2±0.38#+* |
|
Triglycerides, mmol/L |
1.17±0.83 |
1.09±0.34 |
0.74±0.23 |
1.02±0.29 |
1.05±0.32 |
1.37±0.44# |
0.79±0.09 |
Note: * – statistical significance of the difference about the indicators of the group of intact animals at p<0.05; # – statistical significance of the difference in relation to the indicators of the group of animals treated with fusidine at p<0.05; +* – statistical significance of the difference in relation to the indicators of the control group at p<0.05.
The studied dosage forms containing Trigonella foenumgraecum, Cymbopogon proximus, and Acacia nilotica plants prevent the growth of ALT by 34, 35.5, and 38.6%, respectively, p<0.001. Against the background of the use of Cyperus rotundus dosage forms, a decrease in AST activity by 59% is observed, and a statistically significant increase in the level of nitrogenous slags is also observed against the background of fusidine (+18.2%), Trigonella foenumgraecum (+118%), Cyperus rotundus (+137%), p<0.001. It is worth noting that an increase in urea levels was not accompanied by an increase in creatinine, which, of course, indicates not a violation of kidney function, but the induction of protein-synthetic processes and an increase in urea-synthesizing liver function [34, 35].
Only against the background of oral administration of Acacia nilotica aqueous extract, there was a statistically significant increase in albumins by 14%, p<0.001. Against the background of the use of Trigonella foenumgraecum aqueous extract, there was a statistically significant increase in total cholesterol by 49%, p<0.001. Notably, the positive dynamics of metabolic processes and metabolic shifts can be considered as a trigger component of stimulation of regenerative and reparative mechanisms, against the background of phytotherapy [36, 37].
In the general analysis of peripheral blood in animals in the control series using fusidine, leuko-, lymph- and monocytopenia, as well as granulocytosis, were detected. The number of red blood cells increased by 93%, hemoglobin increased by 148%, and a decrease in the number of platelets by 38.8% was also revealed in comparison with the indicators in a series of animals treated with vaseline, p<0.001 (Tables 2 and 3).
|
Table 2. Peripheral blood parameters of white rats with a wound process against the background of dexamethasone solution administration and fusidine treatment (M ±m) |
|||
|
Index |
Groups |
||
|
Intact |
Control (vaseline) |
fusidine |
|
|
Mean concentration hemoglobin. pg/L |
15.48±1.33 |
15.85±65 |
18.22±1.77# |
|
Mean corpuscular hemoglobin concentration, g/L |
263±15.81 |
267.4±16.59 |
293.2±20.83 *# |
|
Mean cell volume, fL |
60.38±2.84 |
59.85±3.02 |
62.3±5.39 |
|
PLT, thrombocytes, ×109/L |
476±12.20 |
474.6±11.21 |
328.25±38.23*# |
|
Red cells distribution, % |
14.32±0.85 |
14.1±1.2 |
14.44±0.21 |
|
Hematocrit, % |
16.72±1.84 |
18.58±3.65 |
41.6±3.84 *# |
|
Hemoglobin, g/L |
98.24±11.83 |
50.6±7.8* |
125.8±16.83 * |
|
Granulocytes, % |
16.24 ± 6.8 |
24.43.7±8.06 |
39.07±6.66 *# |
|
Granulocytes, units/L |
0.362±0.10 |
0.36±0.13 |
0.676±0.1 *# |
|
Leukocytes, ×109/L |
2.48±0.33 |
2.42±0.26 |
0.925±0.15 *# |
|
Lymphocytes, % |
75.84±9.63 |
74.65±11.19 |
31.25±12.25 *# |
|
Lymphocytes, units/L |
1.86±0.29 |
1.85±0.3 |
0.325 ±0.1 *# |
|
Monocytes, units/L |
0.124± 0.04 |
0.14±0.05 |
0.05±0.009 *# |
|
Monocytes, % |
5.35±1.84 |
6.22±1.57 |
7.68±2.2 |
|
Erythrocytes, ×1012/L |
4.02±2.15 |
3.06±0.59 |
6.98±0.41 * |
Note: * – statistical significance of the difference in relation to the indicators of the group of intact animals at p<0.01; # – statistical significance in relation to the indicators of the control group (vaseline) at p<0.01.
Dosage forms containing Cyperus rotundus and Cymbopogon proximus prevent the development of leukopenia in control animals treated with fusidine (an increase in the number of leukocytes by 95% and 215%, respectively). Dosage forms containing Trigonella foenumgraecum and Acacia nilotica did not affect the correction of leukopenia.
|
Table 3. Peripheral blood parameters of white rats with a wound process against the background of dexamethasone solution and fusidine treatment (M ±m) |
||||||
|
Index |
Control (fusidine) |
Cymbopogon proximus |
Cyperus rotundus |
Acacia nilotica |
Trigonella foenum- graecum |
Control (vaseline) |
|
Granulocytes, % |
39.07±6.66*# |
16.46±5.82 |
29.83±7.8+* |
67.73±10.0#+* |
15.93.48#+* |
24.43±8.06 |
|
Granulocytes, units/L |
0.676±0.1*# |
0.23±0.04 +* |
0.6±0.14 # |
0.43±0.17 +* |
0.15±0.05 #+* |
0.36±0.13 |
|
Leukocytes, ×109/L |
0.92±0.15*# |
2.9±0.2 #+* |
1.8±0.56#+* |
0.63±0.2# |
0.95±0.1 # |
2.42±0.26 |
|
Lymphocytes, % |
31.25±12.25*# |
87.05±2.8 #+* |
70.65±3.14+* |
11.51±2.74#+* |
76.77±4.47 +* |
74.65±11.19 |
|
Lymphocytes, units/L |
0.325 ±0.05*# |
1.57±0.47 +* |
1.9±0.53 +* |
0.14±0.06 # |
0.7±0.08 # |
1.85±0.3 |
|
Monocytes, units/L |
0.05±0.009*# |
0.107±0.075 |
0.125±0.05+* |
0.078±0.025 |
0.097±0.005+* |
0.14±0.05 |
|
Monocytes, % |
7.68±2.2 |
1.7±0.63#+* |
3.8±0.49#+* |
10.55±2.82 # |
7.3±1.46 |
6.22±1.57 |
|
Thrombocytes, ×109/л |
328.25±38.23*# |
633.5±160.8 |
2209.71±316 #+* |
1231.75±377#+* |
1040±101.59#+* |
474.6±11.21 |
Note: * – statistical significance of the difference in relation to the indicators of the group of intact animals at p<0.01; # – statistical significance of the difference in relation to the indicators of the group of animals treated with fusidine at p<0.01; +* – statistical significance of the difference in relation to the indicators of the control group (vaseline) at p<0.01.
Emulsions containing Cyperus rotundus and Cymbopogon proximus lead to lymphocyte growth by 484% and 383%, respectively, but do not correct granulocytopenia and monocytopenia. Acacia nilotica, Trigonella foenumgraecum, and Cyperus rotundus cause an increase in platelet levels by 3.76, 3.2, and 6.72 times, respectively, p<0.01. The use of dosage forms containing Cymbopogon proximus, Acacia nilotica, Trigonella foenumgraecum, and Cyperus rotundus contributed to maintaining a high level of erythrocytes, which was comparable to those in the fusidine series (control), statistically significantly higher than in the vaseline series, p<0.01 (Table 4).
|
Table 4. Peripheral blood parameters of white rats with a wound process against the background of the use of dexamethasone solution and treatment with dosage forms based on Acacia nilotica, Cymbopogon proximus, Trigonella foenumgraecum, Cyperus rotundus (M ± m) |
||||||
|
Index |
Control (fusidine) |
Acacia nilotica |
Cymbopogon proximus |
Trigonella foenum- graecum |
Cyperus rotundus |
Control (vaseline) |
|
Mean concentration hemoglobin. pg/L |
18.22±1.77+* |
14.47±1.82 # |
18.37±1.02+* |
18.45±2.25+* |
18.77±3.35 |
15.85±65 |
|
Mean corpuscular hemoglobin concentration, g/L |
293.2±20.11*+* |
269±44.50#+* |
286.5±16.2#+* |
291.25±22.4#+* |
331.5±58.0#+* |
267.4±16.5 |
|
Mean cell volume, fL |
62.3±5.39 |
54.4±2.59#+* |
64.575±7.22 |
63.825±9.8 |
56.8±0.75+* |
59.85±3.02 |
|
Red cells distribution, % |
14.44±0.21 |
15.52±0.94#+* |
17.05±2.88+* |
14.72±1.15 |
12.2±0.80#+* |
14.1±1.2 |
|
Hematocrit, % |
41.6±3.84*+* |
22.2±5.53 # |
38.1±5.64+* |
40.55±11.08+* |
36.3±14+* |
18.58±3.65 |
|
Hemoglobin, g/L |
125.8±16.83+* |
45.75±7.22# |
109.5±20+* |
116.75±26.17+* |
134.5±14.70+* |
50.6±7.51* |
|
Erythrocytes, ×1012/L |
6.98±0.41+* |
5.39±1.8+* |
6.0±1.40+* |
6.44±2.19+* |
7.03±1.67+* |
3.06±0.59 |
Note: * – statistical significance of the difference in relation to the indicators of the group of intact animals at p<0.01; # – statistical significance of the difference in relation to the indicators of the group of animals treated with fusidine at p<0.01; +* – statistical significance of the difference in relation to the indicators of the control group (vaseline) at p<0.01.
Dosage forms containing Cyperus rotundus, Cymbopogon proximus, Trigonella foenumgraecum, and Acacia nilotica act as stimulators of regeneration processes in the wound. The effect on regeneration processes in conditions of dexamethasone-induced hyperglycemia is comparable to the effect of fusidine [38, 39]. The dosage forms of the studied herbs had a positive effect on the studied components of the wound process similar to the local application of ointment with fusidine [40].
The antibacterial activity of the components of Acacia nilotica, Trigonella foenumgraecum, Cyperus rotundus, and Cymbopogon proximus provides a positive effect and promotes regeneration processes [41, 42]. There is pronounced immunomodulating, hepatoprotective, antioxidant, and antitoxic activity in studied herbs, which corresponds to the results of other researchers [43-45]. For instance, Cyperus rotundus contains alkaloids, tannins, flavonoids, furochromones, monoterpenes, glycosides, sesquiterpenes, saponins, sitosterol, essential oils, protein, carbohydrates, terpenoids, starch, separated amino acids and other secondary metabolites [46, 47]. Trigonella foenumgraecum has a high nutritional value, which makes it possible to use this plant as a permanent food product, which also helps to mobilize the body's defenses [48, 49].
CONCLUSION
The use of aqueous emulsions containing Acacia nilotica, Trigonella foenumgraecum, Cyperus rotundus, and Cymbopogon proximus has a positive effect on dysmetabolic disorders that occur during experimental dexamethasone-induced hyperglycemia (activity of hepatic transaminases decreases, blood glucose levels normalize). Peripheral blood composition was optimized (erythrocyte and hemoglobin levels increased, and leukopenia was leveled). Such shifts create a favorable background for the course of reparative processes against the background of dexamethasone-induced damage, which inhibits the course of regenerative processes. Thus, the use of Caucasus herbs can be recommended for diabetes mellitus.
ACKNOWLEDGMENTS: None
CONFLICT OF INTEREST: None
FINANCIAL SUPPORT: None
ETHICS STATEMENT: The protocol for experiments with laboratory animals complied with the requirements of the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes and approved by the Ethics Commission (protocol 3 dated by Aug 3, 2024).
References
- Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Curr Vasc Pharmacol. 2020;18(2):104-9. doi:10.2174/1570161117666190405165911
- Lima-Martínez MM, Carrera Boada C, Madera-Silva MD, Marín W, Contreras M. COVID-19, and diabetes: A bidirectional relationship. Clin Investig Arterioscler. 2021;33(3):151-7. doi:10.1016/j.arteri.2020.10.001
- Lin R, Brown F, James S, Jones J, Ekinci E. Continuous glucose monitoring: A review of the evidence in type 1 and 2 diabetes mellitus. Diabet Med. 2021;38(5):e14528. doi:10.1111/dme.14528
- Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina (Kaunas). 2021;57(10):1072. doi:10.3390/medicina57101072
- Wilkinson HN, Hardman MJ. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223
- Dong J, Chen L, Zhang Y, Jayaswal N, Mezghani I, Zhang W, et al. Mast cells in diabetes and diabetic wound healing. Adv Ther. 2020;37(11):4519-37. doi:10.1007/s12325-020-01499-4
- Martín-Peláez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients. 2020;12(8):2236. doi:10.3390/nu12082236
- Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and healthy lifestyle in the management of gestational diabetes mellitus. Nutrients. 2020;12(10):3050. doi:10.3390/nu12103050
- Zhou C, Wang M, Liang J, He G, Chen N. Ketogenic diet benefits to weight loss, glycemic control, and lipid profiles in overweight patients with type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Int J Environ Res Public Health. 2022;19(16):10429. doi:10.3390/ijerph191610429
- Dashti HM, Mathew TC, Al-Zaid NS. Efficacy of low-carbohydrate ketogenic diet in the treatment of type 2 diabetes. Med Princ Pract. 2021;30(3):223-35. doi:10.1159/000512142
- Toi PL, Anothaisintawee T, Chaikledkaew U, Briones JR, Reutrakul S, Thakkinstian A. Preventive role of diet interventions and dietary factors in type 2 diabetes mellitus: An umbrella review. Nutrients. 2020;12(9):2722. doi:10.3390/nu12092722
- Sharma A, Chawla R, Kaur J, Madaan R. An overview of phytotherapy used in the management of type II Diabetes. Curr Diabetes Rev. 2022;18(6):e170621194148. doi:10.2174/1573399817666210617154535
- Borse SP, Chhipa AS, Sharma V, Singh DP, Nivsarkar M. Management of type 2 diabetes: Current strategies, unfocussed aspects, challenges, and alternatives. Med Princ Pract. 2021;30(2):109-21. doi:10.1159/000511002
- Garima S, Ajit Kumar P, Marcy DM, Sakthivel R, Bhim Pratap S, Nachimuthu Senthil K. Ethnobotanical survey of medicinal plants used in the management of cancer and diabetes. J Tradit Chin Med. 2020;40(6):1007-17. doi:10.19852/j.cnki.jtcm.2020.06.012
- Venkateswaran MR, Vadivel TE, Jayabal S, Murugesan S, Rajasekaran S, Periyasamy S. A review on network pharmacology based phytotherapy in treating diabetes- An environmental perspective. Environ Res. 2021;202:111656. doi:10.1016/j.envres.2021.111656
- Ghorbani A, Mobasheri L, Moshirian Farahi SM, Alavi MS, Fakharzadeh Moghaddam O, Nikpasand N, et al. Type-1 diabetes: Lessons from a decade of preclinical studies on phytotherapy. Fitoterapia. 2024;175:105895. doi:10.1016/j.fitote.2024.105895
- Cheng YO, Veettil SK, Syeed MS, Shetty NY, Gopinath D. Comparative efficacy of therapeutic interventions for the management of recurrent Aphthous ulcers: A systematic review and network meta-analysis. J Evid Based Dent Pract. 2023;23(4):101918. doi:10.1016/j.jebdp.2023.101918
- Butzge JC, Pivotto C, Mezzomo L, Ferrão SK, Picanço JMA, Mezzari A, et al. Antifungal properties of essential oils derived from the genus cymbopogon: A systematic review. Chem Biodivers. 2023;20(10):e202300663. doi:10.1002/cbdv.202300663
- Visuvanathan T, Than LTL, Stanslas J, Chew SY, Vellasamy S. Revisiting trigonella foenum-graecum L.: Pharmacology and therapeutic potentialities. Plants (Basel). 2022;11(11):1450. doi:10.3390/plants11111450
- Xue BX, He RS, Lai JX, Mireku-Gyimah NA, Zhang LH, Wu HH. Phytochemistry, data mining, pharmacology, toxicology and the analytical methods of Cyperus rotundus L. (Cyperaceae): A comprehensive review. Phytochem Rev. 2023:1-46. doi:10.1007/s11101-023-09870-3
- Zhou X, Guo Y, Yang K, Liu P, Wang J. The signaling pathways of traditional Chinese medicine in promoting diabetic wound healing. J Ethnopharmacol. 2022;282:114662. doi:10.1016/j.jep.2021.114662
- Ajebli M, Khan H, Eddouks M. Natural alkaloids and diabetes mellitus: A review. Endocr Metab Immune Disord Drug Targets. 2021;21(1):111-30. doi:10.2174/1871530320666200821124817
- Behl T, Gupta A, Albratty M, Najmi A, Meraya AM, Alhazmi HA, et al. Alkaloidal phytoconstituents for diabetes management: Exploring the unrevealed potential. Molecules. 2022;27(18):5851. doi:10.3390/molecules27185851
- Paul RK, Kesharwani P, Raza K. Recent update on nano-phytopharmaceuticals in the management of diabetes. J Biomater Sci Polym Ed. 2021;32(15):2046-68. doi:10.1080/09205063.2021.1952381
- Sadulaev R, Magomedov T, Khurtueva A, Geteriev A, Turabov N, Koba A, et al. Toxicological assessment of the effect of cadmium chloride on quantitative and qualitative parameters of spermatogenesis in vivo. J Med Pharm Chem Res. 2025;7(2):321-32. doi:10.48309/jmpcr.2025.458716.1257
- Lyashenko EN, Uzbekova LD, Polovinkina VV, Dorofeeva AK, Ibragimov SS, Tatamov AA, et al. Study of the embryonic toxicity of TiO2 and ZrO2 nanoparticles. Micromachines (Basel). 2023;14(2):363. doi:10.3390/mi14020363
- Sugianto AB, Jamil AS, Muchlisin MA. Unveiling the pharmacological potential of Annona squamosa fruit: A network pharmacology approach. J Med Pharm Chem Res. 2025;7(3):410-21. doi:10.48309/jmpcr.2025.454124.1202
- Verevkina M, Goncharov V, Nesmeyanov E, Kamalova O, Baklanov I, Pokhilko A, et al. Application of the Se NPs-Chitosan molecular complex for the correction of selenium deficiency in rats model. Potravinarstvo Slovak J Food Sci. 2023;17:455-66. doi:10.5219/1871
- Sadovoy VV, Selimov M, Shchedrina T, Nagdalian AA. Nutritional supplement for control of diabetes. J Excip Food Chem. 2017;8352017:1843.
- Dzugutova K, Kozyreva Z, Mekhtieva K, Bertaev B, Agkatsev A, Zaseev R, et al. Experimental in vivo evaluation of the activity of hydroxyapatite modified with selenium nanoparticles against caries in laboratory animals. J Med Pharm Chem Res. 2024;7(1):89-97. doi:10.48309/jmpcr.2025.457522.1243
- Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013;43(3):179-94. doi:10.1007/s40279-013-0017-1
- Rzhepakovsky IV, Areshidze DA, Avanesyan SS, Grimm WD, Filatova NV, Kalinin AV, et al. Phytochemical characterization, antioxidant activity, and cytotoxicity of methanolic leaf extract of chlorophytum comosum (Green Type) (Thunb.) Jacq. Molecules. 2022;27(3):762. doi:10.3390/molecules27030762
- Bittner Fialová S, Rendeková K, Mučaji P, Nagy M, Slobodníková L. Antibacterial activity of medicinal plants and their constituents in the context of skin and wound infections, considering european legislation and folk medicine-A review. Int J Mol Sci. 2021;22(19):10746. doi:10.3390/ijms221910746
- Tang XM, Xie MX, Gou JL, Chen L, Tian JL, Zhang X, et al. Antibacterial activity of plants in Cirsium: A comprehensive review. Chin J Integr Med. 2024;30(9):835-41. doi:10.1007/s11655-024-3757-2
- Huang X, Chen X, Xian Y, Jiang F. Anti-virus activity and mechanisms of natural polysaccharides from medicinal herbs. Carbohydr Res. 2024;542:109205. doi:10.1016/j.carres.2024.109205
- Islam Shawon S, Nargis Reyda R, Qais N. Medicinal herbs and their metabolites with biological potential to protect and combat liver toxicity and its disorders: A review. Heliyon. 2024;10(3):e25340. doi:10.1016/j.heliyon.2024.e25340
- Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, et al. Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants (Basel). 2020;9(12):1309. doi:10.3390/antiox9121309
- Taheri Y, Herrera-Bravo J, Huala L, Salazar LA, Sharifi-Rad J, Akram M, et al. Cyperus spp.: A review on phytochemical composition, biological activity, and health-promoting effects. Oxid Med Cell Longev. 2021;2021:4014867. doi:10.1155/2021/4014867
- Wang F, Zhang S, Zhang J, Yuan F. Systematic review of ethnomedicine, phytochemistry, and pharmacology of Cyperi Rhizoma. Front Pharmacol. 2022;13:965902. doi:10.3389/fphar.2022.965902
- Neftullayeva A, Azimova S, Maskurova Y, Tsimgigova R, Papanova A, Dachaeva S, et al. Investigation of the yield of biologically active substances during the ultrasound and electro-discharge extraction of medicinal herbs of the foothills of the north caucasus. Potr S J F Sci. 2023;17:217-30. doi:10.5219/1843
- Skrzypiec-Spring M, Pokrywka A, Kuliczkowska-Płaksej J, Szeląg A, Bolanowski M. Withania somnifera and Trigonella foenum-graecum as ingredients of testosterone-boosting supplements: Possible clinical implications. Adv Clin Exp Med. 2024. doi:10.17219/acem/185743
- Eltayeb LB. Vancomycin-resistant enterococci (VRE) isolated from hospitalized patients: Molecular characterization of the van B gene. J Adv Pharm Educ Res. 2022;12(3-2022):87-92.
- Radhi AA, Jaafar IS. Factors influencing the dissolution behavior of meloxicam dispersions. J Adv Pharm Educ Res. 2022;12(3-2022):9-14.
- Chauhan V, Dalvadi H. A systematic review of spherical agglomeration by particle design of drug formulation. Pharmacophore. 2022;13(1-2022):83-90.
- Chavan YS, Shinkar DM, Jadhav SS, Pingale PL, Boraste SS, Amrutkar SV. A simple glance at the transdermal drug delivery system. Pharmacophore. 2022;13(3-2022):72-80.
- Alsubeie MS. Morphology and molecular study of cassia angustifolia Vahl. in Saudi Arabia using RAPD technique. Entomol Appl Sci Lett. 2022;9(2-2022):11-6.
- Canassa VF, Baldin EL. Nymphal performance and fecundity of melanaphis sacchari (Zehntner)(Hemiptera: Aphididae) in different sorghum genotypes. Entomol Appl Sci Lett. 2022;9(2-2022):1-0.
- Zagade H, Varma S, Suragimath G, Zope S. Knowledge, awareness, and practices of oral health for debilitated patients, among nursing staff of Krishna hospital. Int J Pharm Res Allied Sci. 2022;11(2-2022):73-80.
- Sajini S, Alshouibi E, Balto N, Alkhanbashi A, Abdel-maksoud H. The development and assessment of different shade selection protocols, a novel approach. Int J Pharm Res Allied Sci. 2022;11(2-2022):86-91.